Strategy to Enhance Anticancer Activity and Induced Immunogenic Cell Death of Antimicrobial Peptides by Using Non-Nature Amino Acid Substitutions
- PMID: 35625834
- PMCID: PMC9138567
- DOI: 10.3390/biomedicines10051097
Strategy to Enhance Anticancer Activity and Induced Immunogenic Cell Death of Antimicrobial Peptides by Using Non-Nature Amino Acid Substitutions
Abstract
There is an urgent and imminent need to develop new agents to fight against cancer. In addition to the antimicrobial and anti-inflammatory activities, many antimicrobial peptides can bind to and lyse cancer cells. P-113, a 12-amino acid clinically active histatin-rich peptide, was found to possess anti-Candida activities but showed poor anticancer activity. Herein, anticancer activities and induced immunogenic cancer cell death of phenylalanine-(Phe-P-113), β-naphthylalanine-(Nal-P-113), β-diphenylalanine-(Dip-P-113), and β-(4,4'-biphenyl)alanine-(Bip-P-113) substituted P-113 were studied. Among these peptides, Nal-P-113 demonstrated the best anticancer activity and caused cancer cells to release potent danger-associated molecular patterns (DAMPs), such as reactive oxygen species (ROS), cytochrome c, ATP, and high-mobility group box 1 (HMGB1). These results could help in developing antimicrobial peptides with better anticancer activity and induced immunogenic cell death in therapeutic applications.
Keywords: DAMPs; antimicrobial peptides; bulky non-nature amino acid; cancer; membrane integrity; oncolytic peptides.
Conflict of interest statement
None of the authors have potential conflicts of interest to be disclosed.
Figures
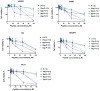


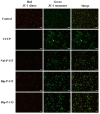



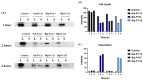

 ) induced tumor cellular lysis through membrane destabilization, caused the release of danger-associated molecular patterns (DAMPs), including ATP (
) induced tumor cellular lysis through membrane destabilization, caused the release of danger-associated molecular patterns (DAMPs), including ATP ( ), HMGB1 (
), HMGB1 ( ), and ROS (
), and ROS ( ), as well as tumor antigens into the tumor microenvironment. Thereafter, these DAMPs recruited the immature dendritic cells (DCs) and further induced the maturation of dendritic cells. The mature DCs were then priming for antigen presentation to T cells and subsequent antitumor immune responses.
), as well as tumor antigens into the tumor microenvironment. Thereafter, these DAMPs recruited the immature dendritic cells (DCs) and further induced the maturation of dendritic cells. The mature DCs were then priming for antigen presentation to T cells and subsequent antitumor immune responses.References
-
- Zharkova M.S., Orlov D.S., Golubeva O.Y., Chakchir O.B., Eliseev I.E., Grinchuk T.M., Shamova O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination with Conventional Antibiotics—A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019;9:128. doi: 10.3389/fcimb.2019.00128. - DOI - PMC - PubMed
-
- Martinez M., Gonçalves S., Felício M.R., Maturana P., Santos N.C., Semorile L., Hollmann A., Maffía P.C. Synergistic and antibiofilm activity of the antimicrobial peptide P5 against carbapenem-resistant Pseudomonas aeruginosa. Biochim. Biophys. Acta. 2019;1861:1329–1337. doi: 10.1016/j.bbamem.2019.05.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

