Lipid Metabolic Alterations in the ALS-FTD Spectrum of Disorders
- PMID: 35625841
- PMCID: PMC9138405
- DOI: 10.3390/biomedicines10051105
Lipid Metabolic Alterations in the ALS-FTD Spectrum of Disorders
Abstract
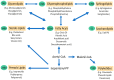
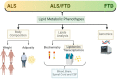
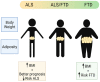
There is an increasing interest in the study of the relation between alterations in systemic lipid metabolism and neurodegenerative disorders, in particular in Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD). In ALS these alterations are well described and evident not only with the progression of the disease but also years before diagnosis. Still, there are some discrepancies in findings relating to the causal nature of lipid metabolic alterations, partly due to the great clinical heterogeneity in ALS. ALS presentation is within a disorder spectrum with Frontotemporal Dementia (FTD), and many patients present mixed forms of ALS and FTD, thus increasing the variability. Lipid metabolic and other systemic metabolic alterations have not been well studied in FTD, or in ALS-FTD mixed forms, as has been in pure ALS. With the recent development in lipidomics and the integration with other -omics platforms, there is now emerging data that not only facilitates the identification of biomarkers but also enables understanding of the underlying pathological mechanisms. Here, we reviewed the recent literature to compile lipid metabolic alterations in ALS, FTD, and intermediate mixed forms, with a view to appraising key commonalities or differences within the spectrum.
Keywords: ALS; FTD; cholesterol; lipid metabolism; lipidomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ghirlanda G., Oradei A., Manto A., Lippa S., Uccioli L., Caputo S., Greco A.V., Littarru G.P. Evidence of Plasma CoQ10-Lowering Effect by HMG-CoA Reductase Inhibitors: A Double-Blind, Placebo-Controlled Study. J. Clin. Pharmacol. 1993;33:226–229. doi: 10.1002/j.1552-4604.1993.tb03948.x. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

