Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury
- PMID: 35625842
- PMCID: PMC9139078
- DOI: 10.3390/biomedicines10051106
Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury
Abstract
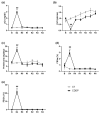
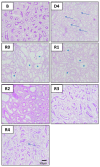
Acute kidney injury (AKI) poses an increased risk factor for new AKI episodes, progression to chronic kidney disease, and death. A worsened evolution has been linked to an incomplete renal repair beyond the apparent functional recovery based on plasma creatinine (pCr) normalization. However, structural sequelae pass largely unnoticed due to the absence of specific diagnostic tools. The urinary kidney injury molecule 1 (KIM-1) participates in renal tissue damage and repair and is proposed as a biomarker of early and subclinical AKI. Thus, we study in this paper the evolution of KIM-1 urinary excretion alongside renal tissue sequelae after an intrinsic AKI episode induced by cisplatin in Wistar rats. Creatinine clearance, pCr, proteinuria and the fractional excretion of Na+ and glucose were used to monitor renal function. Renal tissue damage was blindly scored in kidney specimens stained with hematoxylin-eosin and periodic acid-Schiff. KIM-1 urinary excretion and renal mRNA expression were also assessed. Finally, we analyzed urinary KIM-1 in patients apparently recovered from AKI. Our results show that, after the normalization of the standard markers of glomerular filtration and tubular function, the extent of persistent histological findings of tissue repair correlates with the renal expression and urinary level of KIM-1 in rats. In addition, KIM-1 is also elevated in the urine of a significant fraction of patients apparently recovered from an AKI. Besides its potential utility in the early and subclinical diagnosis of renal damage, this study suggests a new application of urinary KIM-1 in the non-invasive follow-up of renal repair after AKI.
Keywords: KIM-1; acute kidney injury; biomarker; subclinical sequelae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Melo F.D.A.F., Macedo E., Bezerra A.C.F., De Melo W.A.L., Mehta R.L., Burdmann E.D.A., Zanetta D.M.T. A systematic review and meta-analysis of acute kidney injury in the intensive care units of developed and developing countries. PLoS ONE. 2020;15:e0226325. doi: 10.1371/journal.pone.0226325. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

