Adeno-Associated Viruses for Modeling Neurological Diseases in Animals: Achievements and Prospects
- PMID: 35625877
- PMCID: PMC9139062
- DOI: 10.3390/biomedicines10051140
Adeno-Associated Viruses for Modeling Neurological Diseases in Animals: Achievements and Prospects
Abstract
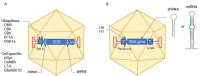
Adeno-associated virus (AAV) vectors have become an attractive tool for efficient gene transfer into animal tissues. Extensively studied as the vehicles for therapeutic constructs in gene therapy, AAVs are also applied for creating animal models of human genetic disorders. Neurological disorders are challenging to model in laboratory animals by transgenesis or genome editing, at least partially due to the embryonic lethality and the timing of the disease onset. Therefore, gene transfer with AAV vectors provides a more flexible option for simulating genetic neurological disorders. Indeed, the design of the AAV expression construct allows the reproduction of various disease-causing mutations, and also drives neuron-specific expression. The natural and newly created AAV serotypes combined with various delivery routes enable differentially targeting neuronal cell types and brain areas in vivo. Moreover, the same viral vector can be used to reproduce the main features of the disorder in mice, rats, and large laboratory animals such as non-human primates. The current review demonstrates the general principles for the development and use of AAVs in modeling neurological diseases. The latest achievements in AAV-mediated modeling of the common (e.g., Alzheimer's disease, Parkinson's disease, ataxias, etc.) and ultra-rare disorders affecting the central nervous system are described. The use of AAVs to create multiple animal models of neurological disorders opens opportunities for studying their mechanisms, understanding the main pathological features, and testing therapeutic approaches.
Keywords: adeno-associated viruses; animal models of human disease; genetic neurological disorders; transgene delivery; viral vectors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., et al. Global, Regional, and National Burden of Neurological Disorders, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459. doi: 10.1016/S1474-4422(18)30499-X. - DOI - PMC - PubMed
-
- Sugarman E.A., Nagan N., Zhu H., Akmaev V.R., Zhou Z., Rohlfs E.M., Flynn K., Hendrickson B.C., Scholl T., Sirko-Osadsa D.A., et al. Pan-Ethnic Carrier Screening and Prenatal Diagnosis for Spinal Muscular Atrophy: Clinical Laboratory Analysis of >72 400 Specimens. Eur. J. Hum. Genet. 2012;20:27–32. doi: 10.1038/ejhg.2011.134. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

