Therapeutic Targeting of Rab GTPases: Relevance for Alzheimer's Disease
- PMID: 35625878
- PMCID: PMC9138223
- DOI: 10.3390/biomedicines10051141
Therapeutic Targeting of Rab GTPases: Relevance for Alzheimer's Disease
Abstract
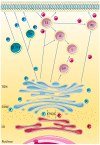
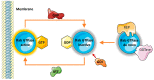
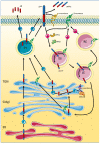
Rab GTPases (Rabs) are small proteins that play crucial roles in vesicle transport and membrane trafficking. Owing to their widespread functions in several steps of vesicle trafficking, Rabs have been implicated in the pathogenesis of several disorders, including cancer, diabetes, and multiple neurodegenerative diseases. As treatments for neurodegenerative conditions are currently rather limited, the identification and validation of novel therapeutic targets, such as Rabs, is of great importance. This review summarises proof-of-concept studies, demonstrating that modulation of Rab GTPases in the context of Alzheimer's disease (AD) can ameliorate disease-related phenotypes, and provides an overview of the current state of the art for the pharmacological targeting of Rabs. Finally, we also discuss the barriers and challenges of therapeutically targeting these small proteins in humans, especially in the context of AD.
Keywords: Alzheimer’s; Rab GTPases; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gallegos M.E., Balakrishnan S., Chandramouli P., Arora S., Azameera A., Babushekar A., Bargoma E., Bokhari A., Chava S.K., Das P., et al. The C. elegans rab family: Identification, classification and toolkit construction. PLoS ONE. 2012;7:e49387. doi: 10.1371/journal.pone.0049387. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

