Altered Ocular Surface Health Status and Tear Film Immune Profile Due to Prolonged Daily Mask Wear in Health Care Workers
- PMID: 35625896
- PMCID: PMC9139140
- DOI: 10.3390/biomedicines10051160
Altered Ocular Surface Health Status and Tear Film Immune Profile Due to Prolonged Daily Mask Wear in Health Care Workers
Abstract
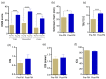
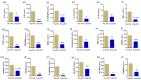
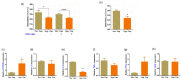
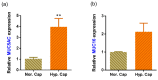
Prolonged daily face mask wearing over several months might affect health of the ocular surface and is reported to be associated with complaints of discomfort and dry-eye-like symptoms. We studied the ocular surface clinical parameters, tear soluble factors and immune cell proportions in ophthalmologists practicing within similar environmental conditions (n = 17) at two time points: pre-face-mask period (Pre-FM; end of 2019) and post-face-mask-wearing period (Post-FM; during 2020 COVID-19 pandemic), with continuous (~8 h/day) mask wear. A significant increase in ocular surface disease index (OSDI) scores without changes in tear breakup time (TBUT), Schirmer's test 1 (ST1) and objective scatter index (OSI) was observed Post-FM. Tear soluble factors (increased-IL-1β, IL-33, IFNβ, NGF, BDNF, LIF and TSLP; decreased-IL-12, IL-13, HGF and VEGF-A) and mucins (MUC5AC) were significantly altered Post-FM. Ex vivo, human donor and corneoscleral explant cultures under elevated CO2 stress revealed that the molecular profile, particularly mucin expression, was similar to the Post-FM tear molecular profile, suggesting hypercapnia is a potential contributor to ocular surface discomfort. Among the immune cell subsets determined from ocular surface wash samples, significantly higher proportions of leukocytes and natural killer T cells were observed in Post-FM compared to Pre-FM. Therefore, it is important to note that the clinical parameters, tear film quality, tear molecular factors and immune cells profile observed in prolonged mask-wear-associated ocular surface discomfort were distinct from dry eye disease or other common ocular surface conditions. These observations are important for differential diagnosis as well as selection of appropriate ocular surface treatment in such subjects.
Keywords: COVID-19; hypercapnia; immune cells; mask; nociception; ocular surface discomfort; soluble factors; tear fluid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) COVID-19 Dashboard. 2021. [(accessed on 8 March 2022)]. Available online: https://coronavirus.jhu.edu/map.html.
Grants and funding
LinkOut - more resources
Full Text Sources

