Sleep Disturbance Alters Cocaine-Induced Locomotor Activity: Involvement of Striatal Neuroimmune and Dopamine Signaling
- PMID: 35625897
- PMCID: PMC9138453
- DOI: 10.3390/biomedicines10051161
Sleep Disturbance Alters Cocaine-Induced Locomotor Activity: Involvement of Striatal Neuroimmune and Dopamine Signaling
Abstract
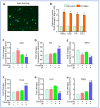
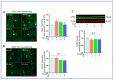
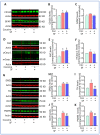
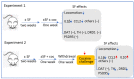
Sleep disorders have high comorbidity with drug addiction and function as major risk factors for developing drug addiction. Recent studies have indicated that both sleep disturbance (SD) and abused drugs could activate microglia, and that increased neuroinflammation plays a critical role in the pathogenesis of both diseases. Whether microglia are involved in the contribution of chronic SDs to drug addiction has never been explored. In this study, we employed a mouse model of sleep fragmentation (SF) with cocaine treatment and examined their locomotor activities, as well as neuroinflammation levels and dopamine signaling in the striatum, to assess their interaction. We also included mice with, or without, SF that underwent cocaine withdrawal and challenge. Our results showed that SF significantly blunted cocaine-induced locomotor stimulation while having marginal effects on locomotor activity of mice with saline injections. Meanwhile, SF modulated the effects of cocaine on neuroimmune signaling in the striatum and in ex vivo isolated microglia. We did not observe differences in dopamine signaling in the striatum among treatment groups. In mice exposed to cocaine and later withdrawal, SF reduced locomotor sensitivity and also modulated neuroimmune and dopamine signaling in the striatum. Taken together, our results suggested that SF was capable of blunting cocaine-induced psychoactive effects through modulating neuroimmune and dopamine signaling. We hypothesize that SF could affect neuroimmune and dopamine signaling in the brain reward circuitry, which might mediate the linkage between sleep disorders and drug addiction.
Keywords: cocaine; dopamine; drug addiction; microglia; neuroinflammation; sleep fragmentation.
Conflict of interest statement
The authors declared no conflict of interests.
Figures




Similar articles
-
Role of Microglia in Psychostimulant Addiction.Curr Neuropharmacol. 2023;21(2):235-259. doi: 10.2174/1570159X21666221208142151. Curr Neuropharmacol. 2023. PMID: 36503452 Free PMC article. Review.
-
Cocaine self-administration differentially activates microglia in the mouse brain.Neurosci Lett. 2020 May 29;728:134951. doi: 10.1016/j.neulet.2020.134951. Epub 2020 Apr 9. Neurosci Lett. 2020. PMID: 32278944 Free PMC article.
-
Cocaine Regulates NLRP3 Inflammasome Activity and CRF Signaling in a Region- and Sex-Dependent Manner in Rat Brain.Biomedicines. 2023 Jun 23;11(7):1800. doi: 10.3390/biomedicines11071800. Biomedicines. 2023. PMID: 37509440 Free PMC article.
-
Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release.Brain Res. 1998 Aug 10;801(1-2):67-71. doi: 10.1016/s0006-8993(98)00546-0. Brain Res. 1998. PMID: 9729284
-
Neuroimmune mechanisms of alcohol and drug addiction.Int Rev Neurobiol. 2014;118:1-12. doi: 10.1016/B978-0-12-801284-0.00001-4. Int Rev Neurobiol. 2014. PMID: 25175859 Free PMC article. Review.
Cited by
-
Role of Microglia in Psychostimulant Addiction.Curr Neuropharmacol. 2023;21(2):235-259. doi: 10.2174/1570159X21666221208142151. Curr Neuropharmacol. 2023. PMID: 36503452 Free PMC article. Review.
References
-
- Bjorness T.E., Greene R.W. Interaction between cocaine use and sleep behavior: A comprehensive review of cocaine’s disrupting influence on sleep behavior and sleep disruptions influence on reward seeking. Pharmacol. Biochem. Behav. 2021;206:173194. doi: 10.1016/j.pbb.2021.173194. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

