Distinct Cellular Tools of Mild Hyperthermia-Induced Acquired Stress Tolerance in Chinese Hamster Ovary Cells
- PMID: 35625909
- PMCID: PMC9138356
- DOI: 10.3390/biomedicines10051172
Distinct Cellular Tools of Mild Hyperthermia-Induced Acquired Stress Tolerance in Chinese Hamster Ovary Cells
Abstract
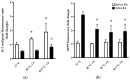
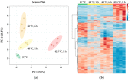
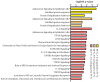
Mild stress could help cells to survive more severe environmental or pathophysiological conditions. In the current study, we investigated the cellular mechanisms which contribute to the development of stress tolerance upon a prolonged (0-12 h) fever-like (40 °C) or a moderate (42.5 °C) hyperthermia in mammalian Chinese Hamster Ovary (CHO) cells. Our results indicate that mild heat triggers a distinct, dose-dependent remodeling of the cellular lipidome followed by the expression of heat shock proteins only at higher heat dosages. A significant elevation in the relative concentration of saturated membrane lipid species and specific lysophosphatidylinositol and sphingolipid species suggests prompt membrane microdomain reorganization and an overall membrane rigidification in response to the fluidizing heat in a time-dependent manner. RNAseq experiments reveal that mild heat initiates endoplasmic reticulum stress-related signaling cascades resulting in lipid rearrangement and ultimately in an elevated resistance against membrane fluidization by benzyl alcohol. To protect cells against lethal, protein-denaturing high temperatures, the classical heat shock protein response was required. The different layers of stress response elicited by different heat dosages highlight the capability of cells to utilize multiple tools to gain resistance against or to survive lethal stress conditions.
Keywords: Chinese hamster ovary cells; acquired stress tolerance; heat shock response; lipidomics; membrane; membrane lipid metabolism; stress; transcriptomics; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- Selye H. Stress without Distress. In: Serban G., editor. Psychopathology of Human Adaptation. Springer; Boston, MA, USA: 1976. pp. 137–146.
-
- Peksel B., Gombos I., Péter M., Vigh L., Tiszlavicz Á., Brameshuber M., Balogh G., Schütz G.J., Horváth I., Vigh L., et al. Mild Heat Induces a Distinct “Eustress” Response in Chinese Hamster Ovary Cells but Does Not Induce Heat Shock Protein Synthesis. Sci. Rep. 2017;7:15643. doi: 10.1038/s41598-017-15821-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

