Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations
- PMID: 35625942
- PMCID: PMC9138220
- DOI: 10.3390/biomedicines10051207
Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations
Abstract
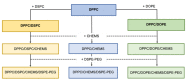
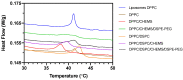
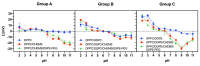
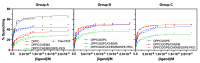
Stimuli-responsive liposomes are a class of nanocarriers whose drug release occurs, preferentially, when exposed to a specific biological environment, to an external stimulus, or both. This work is focused on the design of solid magnetoliposomes (SMLs) as lipid-based nanosystems aiming to obtain multi-stimuli-responsive vesicles for doxorubicin (DOX) controlled release in pathological areas under the action of thermal, magnetic, and pH stimuli. The effect of lipid combinations on structural, colloidal stability, and thermodynamic parameters were evaluated. The results confirmed the reproducibility for SMLs synthesis based on nine lipid formulations (combining DPPC, DSPC, CHEMS, DOPE and/or DSPE-PEG), with structural and colloidal properties suitable for biological applications. A loss of stability and thermosensitivity was observed for formulations containing dioleoylphosphatidylethanolamine (DOPE) lipid. SMLs PEGylation is an essential step to enhance both their long-term storage stability and stealth properties. DOX encapsulation (encapsulation efficiency ranging between 87% and 96%) in the bilayers lowered its pKa, which favors the displacement of DOX from the acyl chains to the surface when changing from alkaline to acidic pH. The release profiles demonstrated a preferential release at acidic pH, more pronounced under mimetic mild-hyperthermia conditions (42 °C). Release kinetics varied with the lipid formulation, generally demonstrating hyperthermia temperatures and acidic pH as determining factors in DOX release; PEGylation was shown to act as a diffusion barrier on the SMLs surface. The integrated assessment and characterization of SMLs allows tuning lipid formulations that best respond to the needs for specific controlled release profiles of stimuli-responsive nanosystems as a multi-functional approach to cancer targeting and therapy.
Keywords: controlled release; doxorubicin; drug delivery; magnetoliposomes; stimuli-responsive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
- UIDB/04650/2020/Fundação para a Ciência e Tecnologia
- UIDB/04436/2020/Fundação para a Ciência e Tecnologia
- UIDP/04436/2020/Fundação para a Ciência e Tecnologia
- PTDC/QUI-QFI/28020/2017/Fundação para a Ciência e Tecnologia
- SFRH/BD/141936/2018/Fundação para a Ciência e Tecnologia
- 2020.02304.CEECIND/Fundação para a Ciência e Tecnologia
- POCI-01-0145-FEDER-028020/Programa Operacional Fatores de Competitividade
- PID2019-106099RB-C43/AEI/10.13039/501100011033/Spanish National Research Council
- PID2019-106099RB-C43/AEI/10.13039/501100011033/European Regional Development Fund
- ELKARTEK program/Ikerbasque
LinkOut - more resources
Full Text Sources

