Comparative Analysis of PSA Density and an MRI-Based Predictive Model to Improve the Selection of Candidates for Prostate Biopsy
- PMID: 35625978
- PMCID: PMC9139805
- DOI: 10.3390/cancers14102374
Comparative Analysis of PSA Density and an MRI-Based Predictive Model to Improve the Selection of Candidates for Prostate Biopsy
Abstract
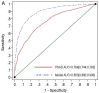
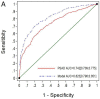
This study is a head-to-head comparison between mPSAD and MRI-PMbdex. The MRI-PMbdex was created from 2432 men with suspected PCa; this cohort comprised the development and external validation cohorts of the Barcelona MRI predictive model. Pre-biopsy 3-Tesla multiparametric MRI (mpMRI) and 2 to 4-core transrectal ultrasound (TRUS)-guided biopsies for suspicious lesions and/or 12-core TRUS systematic biopsies were scheduled. Clinically significant PCa (csPCa), defined as Gleason-based Grade Group 2 or higher, was detected in 934 men (38.4%). The area under the curve was 0.893 (95% confidence interval [CI]: 0.880−0.906) for MRI-PMbdex and 0.764 (95% CI: 0.774−0.783) for mPSAD, with p < 0.001. MRI-PMbdex showed net benefit over biopsy in all men when the probability of csPCa was greater than 2%, while mPSAD did the same when the probability of csPCa was greater than 18%. Thresholds of 13.5% for MRI-PMbdex and 0.628 ng/mL2 for mPSAD had 95% sensitivity for csPCa and presented 51.1% specificity for MRI-PMbdex and 19.6% specificity for mPSAD, with p < 0.001. MRI-PMbdex exhibited net benefit over mPSAD in men with prostate imaging report and data system (PI-RADS) <4, while neither exhibited any benefit in men with PI-RADS 5. Hence, we can conclude that MRI-PMbdex is more accurate than mPSAD for the proper selection of candidates for prostate biopsy among men with suspected PCa, with the exception of men with a PI-RAD S 5 score, for whom neither tool exhibited clinical guidance to determine the need for biopsy.
Keywords: clinically significant prostate cancer; predictive model; prostate-specific antigen density.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Hugosson J., Roobol M.J., Månsson M., Tammela T.L.J., Zappa M., Nelen V., Kwiatkowski M., Lujan M., Carlsson S.V., Talala K.M., et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019;7:643–651. doi: 10.1016/j.eururo.2019.02.009. - DOI - PMC - PubMed
-
- Chou R., Croswell J.M., Dana T., Bougatsos C., Blazina I., Fu R., Gleitsmann K., Koenig H.C., Lam C., Maltz A., et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. - DOI - PubMed
-
- US PSTF. Grossman D.C., Curry S.J., Owens D.K., Bibbins-Domingo K., Caughey A.B., Davidson K.W., Doubeni C.A., Ebell M., Epling J.W., et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.3710. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

