Alterations in Molecular Profiles Affecting Glioblastoma Resistance to Radiochemotherapy: Where Does the Good Go?
- PMID: 35626024
- PMCID: PMC9139489
- DOI: 10.3390/cancers14102416
Alterations in Molecular Profiles Affecting Glioblastoma Resistance to Radiochemotherapy: Where Does the Good Go?
Abstract
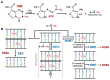
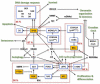
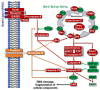
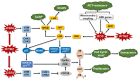
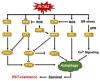
Glioblastoma multiforme (GBM) is a brain tumor characterized by high heterogeneity, diffuse infiltration, aggressiveness, and formation of recurrences. Patients with this kind of tumor suffer from cognitive, emotional, and behavioral problems, beyond exhibiting dismal survival rates. Current treatment comprises surgery, radiotherapy, and chemotherapy with the methylating agent, temozolomide (TMZ). GBMs harbor intrinsic mutations involving major pathways that elicit the cells to evade cell death, adapt to the genotoxic stress, and regrow. Ionizing radiation and TMZ induce, for the most part, DNA damage repair, autophagy, stemness, and senescence, whereas only a small fraction of GBM cells undergoes treatment-induced apoptosis. Particularly upon TMZ exposure, most of the GBM cells undergo cellular senescence. Increased DNA repair attenuates the agent-induced cytotoxicity; autophagy functions as a pro-survival mechanism, protecting the cells from damage and facilitating the cells to have energy to grow. Stemness grants the cells capacity to repopulate the tumor, and senescence triggers an inflammatory microenvironment favorable to transformation. Here, we highlight this mutational background and its interference with the response to the standard radiochemotherapy. We discuss the most relevant and recent evidence obtained from the studies revealing the molecular mechanisms that lead these cells to be resistant and indicate some future perspectives on combating this incurable tumor.
Keywords: acquired resistance; apoptosis; autophagy; cellular homeostasis alterations; malignant glioma; mutational background; radiotherapy; senescence; stemness; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Le Stang N., Bouvier V., Glehen O., Villeneuve L., FRANCIM Network. MESOPATH Referent National Center. Galateau-Salle F., Clin B. Incidence and survival of peritoneal malignant mesothelioma between 1989 and 2015: A population-based study. Cancer Epidemiol. 2019;60:106–111. doi: 10.1016/j.canep.2019.03.014. - DOI - PubMed
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

