Oncological Treatment-Related Fatigue in Oncogeriatrics: A Scoping Review
- PMID: 35626074
- PMCID: PMC9139887
- DOI: 10.3390/cancers14102470
Oncological Treatment-Related Fatigue in Oncogeriatrics: A Scoping Review
Abstract
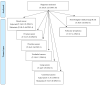
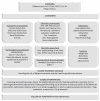
Fatigue is a highly prevalent symptom in both cancer patients and the older population, and it contributes to quality-of-life impairment. Cancer treatment-related fatigue should thus be included in the risk/benefit assessment when introducing any treatment, but tools are lacking to a priori estimate such risk. This scoping review was designed to report the current evidence regarding the frequency of fatigue for the different treatment regimens proposed for the main cancer indications, with a specific focus on age-specific data, for the following tumors: breast, ovary, prostate, urothelium, colon, lung and lymphoma. Fatigue was most frequently reported using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) versions 3 to 5. A total of 324 regimens were analyzed; data on fatigue were available for 217 (67%) of them, and data specific to older patients were available for 35 (11%) of them; recent pivotal trials have generally reported more fatigue grades than older studies, illustrating increasing concern over time. This scoping review presents an easy-to-understand summary that is expected to provide helpful information for shared decisions with patients regarding the anticipation and prevention of fatigue during each cancer treatment.
Keywords: cancer-related fatigue; fatigue; iatrogenic; oncogeriatrics; toxicity.
Conflict of interest statement
Claire Falandry and Rabia Boulahssass reported personal fees from Teva during a dedicated board on the topic. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Rabia Boulahssass reported additional fees from Amgen, Fresenius, Hospira, Lilly, Novartis, Nutricia, Pierre Fabre, Roche, Sanofi, and Takeda outside the submitted work. Claire Falandry reported additional fees from Amgen, Astellas, Astra Zeneca, Baxter, Biogaran, BMS, Chugai, Clovis Oncology, Eisai, Grunenthal, GSK, Hospira, Janssen Oncology, Leo Pharma, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, and Takeda outside the submitted work; grants from Astellas Pharma, Chugai Pharma, Pierre Fabre and Pfizer outside the submitted work; and non-financial support from AstraZeneca, GSK, Janssen Oncology Leo Pharma Novartis and Pierre Fabre outside the submitted work. Other authors declare no conflict of interest.
Figures

References
-
- PDQ Cancer Information Summaries. National Cancer Institute (US); Bethesda, MD, USA: 2002. PDQ Supportive and Palliative Care Editorial Board Fatigue (PDQ®): Health Professional Version. - PubMed
-
- Vogelzang N.J., Breitbart W., Cella D., Curt G.A., Groopman J.E., Horning S.J., Itri L.M., Johnson D.H., Scherr S.L., Portenoy R.K. Patient, Caregiver, and Oncologist Perceptions of Cancer-Related Fatigue: Results of a Tripart Assessment Survey. The Fatigue Coalition. Semin. Hematol. 1997;34:4–12. - PubMed
-
- Abrahams H.J.G., Gielissen M.F.M., Schmits I.C., Verhagen C.A.H.H.V.M., Rovers M.M., Knoop H. Risk Factors, Prevalence, and Course of Severe Fatigue after Breast Cancer Treatment: A Meta-Analysis Involving 12 327 Breast Cancer Survivors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27:965–974. doi: 10.1093/annonc/mdw099. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

