Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma: A Physiopathologic and Pharmacologic Review
- PMID: 35626089
- PMCID: PMC9139729
- DOI: 10.3390/cancers14102486
Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma: A Physiopathologic and Pharmacologic Review
Abstract
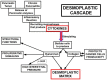
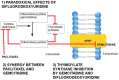
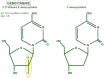
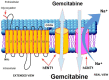
Pancreatic ductal adenocarcinoma (PDAC) is a very aggressive tumor with a poor prognosis and inadequate response to treatment. Many factors contribute to this therapeutic failure: lack of symptoms until the tumor reaches an advanced stage, leading to late diagnosis; early lymphatic and hematic spread; advanced age of patients; important development of a pro-tumoral and hyperfibrotic stroma; high genetic and metabolic heterogeneity; poor vascular supply; a highly acidic matrix; extreme hypoxia; and early development of resistance to the available therapeutic options. In most cases, the disease is silent for a long time, andwhen it does become symptomatic, it is too late for ablative surgery; this is one of the major reasons explaining the short survival associated with the disease. Even when surgery is possible, relapsesare frequent, andthe causes of this devastating picture are the low efficacy ofand early resistance to all known chemotherapeutic treatments. Thus, it is imperative to analyze the roots of this resistance in order to improve the benefits of therapy. PDAC chemoresistance is the final product of different, but to some extent, interconnected factors. Surgery, being the most adequate treatment for pancreatic cancer and the only one that in a few selected cases can achieve longer survival, is only possible in less than 20% of patients. Thus, the treatment burden relies on chemotherapy in mostcases. While the FOLFIRINOX scheme has a slightly longer overall survival, it also produces many more adverse eventsso that gemcitabine is still considered the first choice for treatment, especially in combination with other compounds/agents. This review discusses the multiple causes of gemcitabine resistance in PDAC.
Keywords: desmoplastic reaction; gemcitabine; hydroxyurea; pancreatic ductal adenocarcinoma; proteasome inhibitors; resistance to treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Chemoresistance in pancreatic ductal adenocarcinoma: Overcoming resistance to therapy.Adv Cancer Res. 2023;159:285-341. doi: 10.1016/bs.acr.2023.02.010. Epub 2023 Apr 18. Adv Cancer Res. 2023. PMID: 37268399
-
Current Standard and Future Perspectives in First- and Second-Line Treatment of Metastatic Pancreatic Adenocarcinoma.Digestion. 2016;94(1):44-9. doi: 10.1159/000447739. Epub 2016 Jul 21. Digestion. 2016. PMID: 27438590 Review.
-
Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going.Exp Mol Med. 2017 Dec 1;49(12):e406. doi: 10.1038/emm.2017.255. Exp Mol Med. 2017. PMID: 29611542 Free PMC article. Review.
-
Pancreatic Cancer Chemoresistance to Gemcitabine.Cancers (Basel). 2017 Nov 16;9(11):157. doi: 10.3390/cancers9110157. Cancers (Basel). 2017. PMID: 29144412 Free PMC article. Review.
-
Impact of first-line FOLFIRINOX versus Gemcitabine/Nab-Paclitaxel chemotherapy on survival in advanced pancreatic cancer: Evidence from the prospective international multicentre PURPLE pancreatic cancer registry.Eur J Cancer. 2022 Oct;174:102-112. doi: 10.1016/j.ejca.2022.06.042. Epub 2022 Aug 18. Eur J Cancer. 2022. PMID: 35988408
Cited by
-
Elucidation of the Gemcitabine Transporters of Escherichia coli K-12 and Gamma-Proteobacteria Linked to Gemcitabine-Related Chemoresistance.Int J Mol Sci. 2024 Jun 27;25(13):7012. doi: 10.3390/ijms25137012. Int J Mol Sci. 2024. PMID: 39000123 Free PMC article.
-
Selective and Concentrative Enteropancreatic Recirculation of Antibiotics by Pigs.Antibiotics (Basel). 2023 Dec 21;13(1):12. doi: 10.3390/antibiotics13010012. Antibiotics (Basel). 2023. PMID: 38275322 Free PMC article.
-
Emerging Therapeutic Options in Pancreatic Cancer Management.Int J Mol Sci. 2024 Feb 5;25(3):1929. doi: 10.3390/ijms25031929. Int J Mol Sci. 2024. PMID: 38339207 Free PMC article.
-
Nanoparticle-Mediated Therapy with miR-198 Sensitizes Pancreatic Cancer to Gemcitabine Treatment through Downregulation of VCP-Mediated Autophagy.Pharmaceutics. 2023 Jul 28;15(8):2038. doi: 10.3390/pharmaceutics15082038. Pharmaceutics. 2023. PMID: 37631252 Free PMC article.
-
Targeting ABC transporters in PDAC - past, present, or future?Oncotarget. 2024 Jun 20;15:403-406. doi: 10.18632/oncotarget.28597. Oncotarget. 2024. PMID: 38900606 Free PMC article. No abstract available.
References
-
- Seer Cancer Stat Facts: Pancreas Cancer. [(accessed on 10 February 2022)]; Available online: https://seer.cancer.gov/statfacts/html/pancreas.html.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

