In Vivo Monitoring of Corneal Dendritic Cells in the Subbasal Nerve Plexus during Trastuzumab and Paclitaxel Breast Cancer Therapy-A One-Year Follow-Up
- PMID: 35626335
- PMCID: PMC9139605
- DOI: 10.3390/diagnostics12051180
In Vivo Monitoring of Corneal Dendritic Cells in the Subbasal Nerve Plexus during Trastuzumab and Paclitaxel Breast Cancer Therapy-A One-Year Follow-Up
Abstract
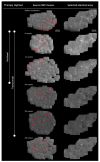
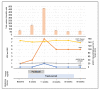
Paclitaxel and trastuzumab have been associated with adverse effects including chemotherapy-induced peripheral neuropathy (CIPN) or ocular complications. In vivo confocal laser scanning microscopy (CLSM) of the cornea could be suitable for assessing side effects since the cornea is susceptible to, i.e., neurotoxic stimuli. The study represents a one-year follow-up of a breast cancer patient including large-area in vivo CLSM of the subbasal nerve plexus (SNP), nerve function testing, and questionnaires during paclitaxel and trastuzumab therapy. Six monitoring sessions (one baseline, four during, and one after therapy) over 58 weeks were carried out. Large-area mosaics of the SNP were generated, and identical regions within all sessions were assigned. While corneal nerve morphology did not cause alterations, the number of dendritic cells (DCs) showed dynamic changes with a local burst at 11 weeks after baseline. Simultaneously, paclitaxel treatment was terminated due to side effects, which, together with DCs, returned to normal levels as the therapy progressed. Longitudinal in vivo CLSM of the SNP could complement routine examinations and be helpful to generate a comprehensive clinical picture. The applied techniques, with corneal structures acting as biomarkers could represent a diagnostic tool for the objective assessment of the severity of adverse events and the outcome.
Keywords: breast cancer; chemotherapy-induced peripheral neuropathy (CIPN); corneal nerves; corneal subbasal nerve plexus (SNP); dendritic cells; identical areas; in vivo large-area confocal laser scanning microscopy (CLSM) of the cornea; one-year follow-up; paclitaxel and trastuzumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Targeted Cancer Therapies Fact Sheet—National Cancer Institute. [(accessed on 14 March 2022)]; Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/t....
-
- Breast Cancer. [(accessed on 23 February 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
Grants and funding
LinkOut - more resources
Full Text Sources

