Diagnostic Accuracy of Point-of-Care HCV Viral Load Assays for HCV Diagnosis: A Systematic Review and Meta-Analysis
- PMID: 35626411
- PMCID: PMC9141110
- DOI: 10.3390/diagnostics12051255
Diagnostic Accuracy of Point-of-Care HCV Viral Load Assays for HCV Diagnosis: A Systematic Review and Meta-Analysis
Abstract
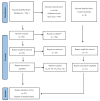
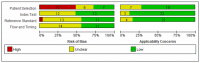
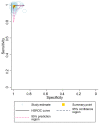
Despite the widespread availability of curative treatment with direct-acting antivirals, a significant proportion of people with HCV remain undiagnosed and untreated. New point-of-care (PoC) HCV RNA assays that can be used in clinical settings may help expand access to testing and treatment. This study aimed to evaluate the diagnostic performance of PoC HCV viral load assays compared to laboratory-based testing. Methods: We searched three databases for studies published before May 2021 that evaluated PoC HCV RNA assays against a laboratory NAT reference standard (Prospero CRD42021269022). Random effects bivariate models were used to summarize the estimates. Stratified analyses were performed based on geographic region, population (PWID, etc.), and specimen type (serum/plasma or fingerstick; fresh or frozen). We used the GRADE approach to assess the certainty of the evidence. Results: A total of 25 studies were eligible. We evaluated five different commercially available viral load assays. The pooled sensitivity and specificity were 99% (95% CI: 98−99%) and 99% (95% CI: 99−100%), respectively. High sensitivity and specificity were observed across different assays, study settings (including LMICs and HICs), and populations. There was a small but statistically significant reduction in sensitivity for fingersticks compared to serum or plasma samples (98% vs. 100%, p < 0.05), but the specificity was similar between frozen and fresh samples. The evidence was rated as moderate-high certainty. Conclusions: PoC HCV viral load assays demonstrate excellent diagnostic performance in various settings and populations. The WHO now recommends using PoC HCV viral load assays as an additional strategy to promote access to confirmatory viral load testing and treatment.
Keywords: diagnostic accuracy; hepatitis c; point-of-care; viral load testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Impact of hepatitis C virus point-of-care RNA viral load testing compared with laboratory-based testing on uptake of RNA testing and treatment, and turnaround times: a systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2023 Mar;8(3):253-270. doi: 10.1016/S2468-1253(22)00346-6. Epub 2023 Jan 24. Lancet Gastroenterol Hepatol. 2023. PMID: 36706775 Free PMC article.
-
Diagnostic Accuracy of Assays Using Point-of-Care Testing or Dried Blood Spot Samples for the Determination of Hepatitis C Virus RNA: A Systematic Review.J Infect Dis. 2022 Sep 21;226(6):1005-1021. doi: 10.1093/infdis/jiac049. J Infect Dis. 2022. PMID: 35150578
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
In-field evaluation of Xpert® HCV viral load Fingerstick assay in people who inject drugs in Tanzania.Liver Int. 2020 Mar;40(3):514-521. doi: 10.1111/liv.14315. Epub 2019 Dec 15. Liver Int. 2020. PMID: 31778282 Free PMC article.
-
Reflex Hepatitis C Virus Viral Load Testing Following an Initial Positive Hepatitis C Virus Antibody Test: A Global Systematic Review and Meta-analysis.Clin Infect Dis. 2023 Oct 13;77(8):1137-1156. doi: 10.1093/cid/ciad126. Clin Infect Dis. 2023. PMID: 37648655
Cited by
-
Impact of storage time in dried blood samples (DBS) and dried plasma samples (DPS) for point-of-care hepatitis C virus (HCV) RNA quantification and HCV core antigen detection.Microbiol Spectr. 2023 Sep 1;11(5):e0174823. doi: 10.1128/spectrum.01748-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37655908 Free PMC article.
-
Independent assessment of a point of care HCV RNA test by laboratory analytical testing and a prospective field study in the U.S.PLoS One. 2025 Jul 22;20(7):e0324088. doi: 10.1371/journal.pone.0324088. eCollection 2025. PLoS One. 2025. PMID: 40694587 Free PMC article.
-
Determination of hepatitis C virus subtype prevalent in Sindh, Pakistan: a phylogenetic analysis.Sci Rep. 2024 May 15;14(1):11159. doi: 10.1038/s41598-024-59342-7. Sci Rep. 2024. PMID: 38750152 Free PMC article.
-
Sustained virological response in HCV patients receiving antiviral treatment at a teaching centre of northern India.J Family Med Prim Care. 2025 Mar;14(3):942-946. doi: 10.4103/jfmpc.jfmpc_1379_24. Epub 2025 Mar 25. J Family Med Prim Care. 2025. PMID: 40256099 Free PMC article.
-
Universal screening for HCV infection in China: An effectiveness and cost-effectiveness analysis.JHEP Rep. 2024 Jan 11;6(4):101000. doi: 10.1016/j.jhepr.2024.101000. eCollection 2024 Apr. JHEP Rep. 2024. PMID: 38481389 Free PMC article.
References
-
- WHO . Global Progress Report on Hiv, Viral Hepatitis And Sexually Transmitted Infections, 2021. WHO; Geneva, Switzerland: 2021.
-
- WHO . Global Health Sector Strategu on Viral Hepatitis 2016–2021. WHO; Geneva, Switzerland: 2016.
-
- WHO . WHO Global Hepatitis Report 2017. World Health Organization; Geneva, Switzerland: 2017.
-
- Oru E., Trickey A., Shirali R., Kanters S., Easterbrook P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: A global systematic review and meta-analysis. Lancet Glob. Health. 2021;9:e431–e445. doi: 10.1016/S2214-109X(20)30505-2. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

