Fluorescence In Situ Hybridization (FISH) Tests for Identifying Protozoan and Bacterial Pathogens in Infectious Diseases
- PMID: 35626441
- PMCID: PMC9141552
- DOI: 10.3390/diagnostics12051286
Fluorescence In Situ Hybridization (FISH) Tests for Identifying Protozoan and Bacterial Pathogens in Infectious Diseases
Abstract
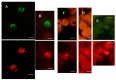
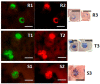
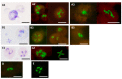
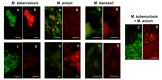
Diagnosing and treating many infectious diseases depends on correctly identifying the causative pathogen. Characterization of pathogen-specific nucleic acid sequences by PCR is the most sensitive and specific method available for this purpose, although it is restricted to laboratories that have the necessary infrastructure and finance. Microscopy, rapid immunochromatographic tests for antigens, and immunoassays for detecting pathogen-specific antibodies are alternative and useful diagnostic methods with different advantages and disadvantages. Detection of ribosomal RNA molecules in the cytoplasm of bacterial and protozoan pathogens by fluorescence in-situ hybridization (FISH) using sequence-specific fluorescently labelled DNA probes, is cheaper than PCR and requires minimal equipment and infrastructure. A LED light source attached to most laboratory light microscopes can be used in place of a fluorescence microscope with a UV lamp for FISH. A FISH test hybridization can be completed in 30 min at 37 °C and the whole test in less than two hours. FISH tests can therefore be rapidly performed in both well-equipped and poorly-resourced laboratories. Highly sensitive and specific FISH tests for identifying many bacterial and protozoan pathogens that cause disease in humans, livestock and pets are reviewed, with particular reference to parasites causing malaria and babesiosis, and mycobacteria responsible for tuberculosis.
Keywords: Babesia duncani; Babesia microti; FISH tests; LED fluorescence microscopy; Mycobacterium avium; Mycobacterium tuberculosis; Plasmodium falciparum; Plasmodium knowlesi; Plasmodium vivax; diagnostic tests; fluorescence in situ hybridization; pathogen identification; ribosomal RNA.
Conflict of interest statement
JSS is stockholder and RR is science advisor at IGeneX Inc., and IDFISH Inc.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

