New Variant in Placophilin-2 Gene Causing Arrhythmogenic Myocardiopathy
- PMID: 35627167
- PMCID: PMC9141741
- DOI: 10.3390/genes13050782
New Variant in Placophilin-2 Gene Causing Arrhythmogenic Myocardiopathy
Abstract
Introduction: Arrhythmogenic cardiomyopathy (ACM) is an inherited disease characterized by progressive fibroadipose replacement of cardiomyocytes. Its diagnosis is based on imaging, electrocardiographic, histological and genetic/familial criteria. The development of the disease is based mainly on desmosomal genes. Knowledge of the phenotypic expression of each of these genes will help in both diagnosis and prognosis. The objective of this study is to describe the genotype-phenotype association of an unknown PKP2 gene variant in a family diagnosed with ACM.
Methods: Clinical and genetic study of a big family carrying the p.Tyr168* variant in the PKP2 gene, in order to demonstrate pathogenicity of this variant, causing ACM.
Results: Twenty-two patients (proband and relatives) were evaluated. This variant presented with high arrhythmic load at an early age, but without evidence of structural heart disease after 20 years of follow-up, with low risk in predictive scores. We demonstrate evidence of its pathogenicity.
Conclusions: The p.Tyr168* variant in the PKP2 gene causes ACM with a high arrhythmic load and with an absence of structural heart disease. This fact emphasizes the value of knowing the phenotypic expression of each variant.
Keywords: CVD genetics; NGS for diagnostics of CVDs-; cardiomyopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

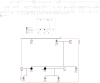
 arrhythmogenic cardiomyopathy—clinically affected.
arrhythmogenic cardiomyopathy—clinically affected.  arrhythmogenic cardiomyopathy—asymptomatic carrier, could later manifest disease.
arrhythmogenic cardiomyopathy—asymptomatic carrier, could later manifest disease.  arrhythmogenic cardiomyopathy—uncertain, possibly affected.
arrhythmogenic cardiomyopathy—uncertain, possibly affected.  arrhythmogenic cardiomyopathy—Not affected. E1 Genetic study: (− negative)/(+ positive). E2 Echocardiogram: (− negative)/(+ positive).
arrhythmogenic cardiomyopathy—Not affected. E1 Genetic study: (− negative)/(+ positive). E2 Echocardiogram: (− negative)/(+ positive).




References
-
- Ripoll-Vera T., Luengo C.P., Alcázar J.C.B., Ruiz A.B.G., Del Valle N.S., Martín B.B., García J.L.P., Buitrago G.G., Martínez C.D., Villena J.C.C., et al. Sudden Cardiac Death in Persons Aged 50 Years or Younger: Diagnostic Yield of a Regional Molecular Autopsy Program Using Massive Sequencing. Rev. Esp. Cardiol. 2020;74:402–413. doi: 10.1016/j.recesp.2020.03.001. - DOI - PubMed
-
- Towbin J.A., McKenna W.J., Abrams D., Ackerman M.J., Calkins H., Darrieux F., Daubert J.P., De Chillou C., DePasquale E.C., Desai M.Y., et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. - DOI - PubMed
-
- Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed Modification of the Task Force Criteria. Eur. Heart J. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

