Revisiting the Roles of Filaggrin in Atopic Dermatitis
- PMID: 35628125
- PMCID: PMC9140947
- DOI: 10.3390/ijms23105318
Revisiting the Roles of Filaggrin in Atopic Dermatitis
Abstract
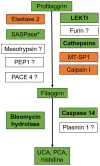
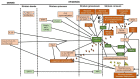
The discovery in 2006 that loss-of-function mutations in the filaggrin gene (FLG) cause ichthyosis vulgaris and can predispose to atopic dermatitis (AD) galvanized the dermatology research community and shed new light on a skin protein that was first identified in 1981. However, although outstanding work has uncovered several key functions of filaggrin in epidermal homeostasis, a comprehensive understanding of how filaggrin deficiency contributes to AD is still incomplete, including details of the upstream factors that lead to the reduced amounts of filaggrin, regardless of genotype. In this review, we re-evaluate data focusing on the roles of filaggrin in the epidermis, as well as in AD. Filaggrin is important for alignment of keratin intermediate filaments, control of keratinocyte shape, and maintenance of epidermal texture via production of water-retaining molecules. Moreover, filaggrin deficiency leads to cellular abnormalities in keratinocytes and induces subtle epidermal barrier impairment that is sufficient enough to facilitate the ingress of certain exogenous molecules into the epidermis. However, although FLG null mutations regulate skin moisture in non-lesional AD skin, filaggrin deficiency per se does not lead to the neutralization of skin surface pH or to excessive transepidermal water loss in atopic skin. Separating facts from chaff regarding the functions of filaggrin in the epidermis is necessary for the design efficacious therapies to treat dry and atopic skin.
Keywords: NMF; atopic dermatitis; epidermis; filaggrin; microbiome; pH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dale B.A., Lonsdale-Eccles J.D., Holbrook K.A. Stratum corneum basic protein: An interfilamentous matrix protein of epidermal keratin. Curr. Probl. Dermatol. 1980;10:311–325. - PubMed
-
- Dale B.A., Presland R.B., Lewis S.P., Underwood R.A., Fleckman P. Transient expression of epidermal filaggrin in cultured cells causes collapse of intermediate filament networks with alteration of cell shape and nuclear integrity. J. Investig. Dermatol. 1997;108:179–187. doi: 10.1111/1523-1747.ep12334205. - DOI - PubMed
-
- Pendaries V., Malaisse J., Pellerin L., Le Lamer M., Nachat R., Kezic S., Schmitt A.M., Paul C., Poumay Y., Serre G., et al. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J. Investig. Dermatol. 2014;134:2938–2946. doi: 10.1038/jid.2014.259. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

