Exogenous dsRNA Induces RNA Interference of a Chalcone Synthase Gene in Arabidopsis thaliana
- PMID: 35628133
- PMCID: PMC9142100
- DOI: 10.3390/ijms23105325
Exogenous dsRNA Induces RNA Interference of a Chalcone Synthase Gene in Arabidopsis thaliana
Abstract
Recent investigations have shown the possibility of artificial induction of RNA interference (RNAi) via plant foliar treatments with naked double-stranded RNA (dsRNA) to silence essential genes in plant fungal pathogens or to target viral RNAs. Furthermore, several studies have documented the downregulation of plant endogenous genes via external application of naked gene-specific dsRNAs and siRNAs to the plant surfaces. However, there are limited studies on the dsRNA processing and gene silencing mechanisms after external dsRNA application. Such studies would assist in the development of innovative tools for crop improvement and plant functional studies. In this study, we used exogenous gene-specific dsRNA to downregulate the gene of chalcone synthase (CHS), the key enzyme in the flavonoid/anthocyanin biosynthesis pathway, in Arabidopsis. The nonspecific NPTII-dsRNA encoding the nonrelated neomycin phosphotransferase II bacterial gene was used to treat plants in order to verify that any observed effects and processing of AtCHS mRNA were sequence specific. Using high-throughput small RNA (sRNA) sequencing, we obtained six sRNA-seq libraries for plants treated with water, AtCHS-dsRNA, or NPTII-dsRNA. After plant foliar treatments, we detected the emergence of a large number of AtCHS- and NPTII-encoding sRNAs, while there were no such sRNAs after control water treatment. Thus, the exogenous AtCHS-dsRNAs were processed into siRNAs and induced RNAi-mediated AtCHS gene silencing. The analysis showed that gene-specific sRNAs mapped to the AtCHS and NPTII genes unevenly with peak read counts at particular positions, involving primarily the sense strand, and documented a gradual decrease in read counts from 17-nt to 30-nt sRNAs. Results of the present study highlight a significant potential of exogenous dsRNAs as a promising strategy to induce RNAi-based downregulation of plant gene targets for plant management and gene functional studies.
Keywords: RNA interference; exogenous dsRNA; gene silencing; plant foliar treatment; plant gene regulation; small RNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
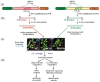
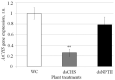
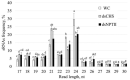
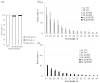
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

