Decreased Tissue Omega-6/Omega-3 Fatty Acid Ratio Prevents Chemotherapy-Induced Gastrointestinal Toxicity Associated with Alterations of Gut Microbiome
- PMID: 35628140
- PMCID: PMC9140600
- DOI: 10.3390/ijms23105332
Decreased Tissue Omega-6/Omega-3 Fatty Acid Ratio Prevents Chemotherapy-Induced Gastrointestinal Toxicity Associated with Alterations of Gut Microbiome
Abstract
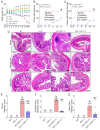
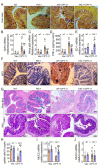
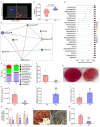
Gastrointestinal toxicity (GIT) is a debilitating side effect of Irinotecan (CPT-11) and limits its clinical utility. Gut dysbiosis has been shown to mediate this side effect of CPT-11 by increasing gut bacterial β-glucuronidase (GUSB) activity and impairing the intestinal mucosal barrier (IMB). We have recently shown the opposing effects of omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) on the gut microbiome. We hypothesized that elevated levels of tissue n-3 PUFA with a decreased n-6/n-3 PUFA ratio would reduce CPT-11-induced GIT and associated changes in the gut microbiome. Using a unique transgenic mouse (FAT-1) model combined with dietary supplementation experiments, we demonstrate that an elevated tissue n-3 PUFA status with a decreased n-6/n-3 PUFA ratio significantly reduces CPT-11-induced weight loss, bloody diarrhea, gut pathological changes, and mortality. Gut microbiome analysis by 16S rRNA gene sequencing and QIIME2 revealed that improvements in GIT were associated with the reduction in the CPT-11-induced increase in both GUSB-producing bacteria (e.g., Enterobacteriaceae) and GUSB enzyme activity, decrease in IMB-maintaining bacteria (e.g., Bifidobacterium), IMB dysfunction and systemic endotoxemia. These results uncover a host-microbiome interaction approach to the management of drug-induced gut toxicity. The prevention of CPT-11-induced gut microbiome changes by decreasing the tissue n-6/n-3 PUFA ratio could be a novel strategy to prevent chemotherapy-induced GIT.
Keywords: CPT-11; FAT-1 transgenic mouse; GUSB; beta-glucuronidase; chemotherapy-induced gut-toxicity; gut microbiome; irinotecan; omega-3 fatty acids; omega-6/omega-3 PUFA ratio.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures








References
-
- Jinno H., Tanaka-Kagawa T., Hanioka N., Saeki M., Ishida S., Nishimura T., Ando M., Saito Y., Ozawa S., Sawada J. Glucuronidation of 7-ethyl-10-hydroxycamptothecin (SN-38), an active metabolite of irinotecan (CPT-11), by human UGT1A1 variants, G71R, P229Q, and Y486D. Drug Metab. Dispos. 2003;31:108–113. doi: 10.1124/dmd.31.1.108. - DOI - PubMed
-
- Mathijssen R.H., van Alphen R.J., Verweij J., Loos W.J., Nooter K., Stoter G., Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin. Cancer Res. 2001;7:2182–2194. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

