Acute Colon Inflammation Triggers Primary Motor Cortex Glial Activation, Neuroinflammation, Neuronal Hyperexcitability, and Motor Coordination Deficits
- PMID: 35628158
- PMCID: PMC9141031
- DOI: 10.3390/ijms23105347
Acute Colon Inflammation Triggers Primary Motor Cortex Glial Activation, Neuroinflammation, Neuronal Hyperexcitability, and Motor Coordination Deficits
Abstract
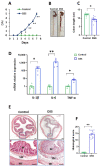
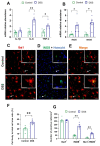
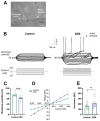
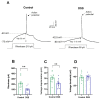
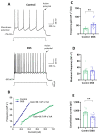
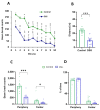
Neuroinflammation underlies neurodegenerative diseases. Herein, we test whether acute colon inflammation activates microglia and astrocytes, induces neuroinflammation, disturbs neuron intrinsic electrical properties in the primary motor cortex, and alters motor behaviors. We used a rat model of acute colon inflammation induced by dextran sulfate sodium. Inflammatory mediators and microglial activation were assessed in the primary motor cortex by PCR and immunofluorescence assays. Electrophysiological properties of the motor cortex neurons were determined by whole-cell patch-clamp recordings. Motor behaviors were examined using open-field and rotarod tests. We show that the primary motor cortex of rats with acute colon inflammation exhibited microglial and astrocyte activation and increased mRNA abundance of interleukin-6, tumor necrosis factor-alpha, and both inducible and neuronal nitric oxide synthases. These changes were accompanied by a reduction in resting membrane potential and rheobase and increased input resistance and action potential frequency, indicating motor neuron hyperexcitability. In addition, locomotion and motor coordination were impaired. In conclusion, acute colon inflammation induces motor cortex microglial and astrocyte activation and inflammation, which led to neurons' hyperexcitability and reduced motor coordination performance. The described disturbances resembled some of the early features found in amyotrophic lateral sclerosis patients and animal models, suggesting that colon inflammation might be a risk factor for developing this disease.
Keywords: colon inflammation; hyperexcitability; microglial and astrocyte activation; motor coordination; motor neurons; neurodegeneration; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kempuraj D., Thangavel R., Selvakumar G.P., Zaheer S., Ahmed M.E., Raikwar S.P., Zahoor H., Saeed D., Natteru P.A., Iyer S., et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017;11:216. doi: 10.3389/fncel.2017.00216. - DOI - PMC - PubMed

