Cannabidiol Enhances Microglial Beta-Amyloid Peptide Phagocytosis and Clearance via Vanilloid Family Type 2 Channel Activation
- PMID: 35628181
- PMCID: PMC9140666
- DOI: 10.3390/ijms23105367
Cannabidiol Enhances Microglial Beta-Amyloid Peptide Phagocytosis and Clearance via Vanilloid Family Type 2 Channel Activation
Abstract
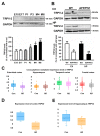
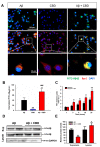
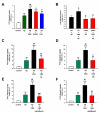
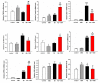
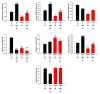
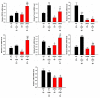
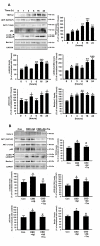
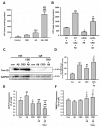
Alzheimer's disease (AD) is associated with the accumulation and aggregation of amyloid in the brain. The cation channel TRPV2 may mediate the pathological changes in mild cognitive impairment. A high-affinity agonist of TRPV2 named cannabidiol is one of the candidate drugs for AD. However, the molecular mechanism of cannabidiol via TRPV2 in AD remains unknown. The present study investigated whether cannabidiol enhances the phagocytosis and clearance of microglial Aβ via the TRPV2 channel. We used a human dataset, mouse primary neuron and microglia cultures, and AD model mice to evaluate TRPV2 expression and the ability of microglial amyloid-β phagocytosis in vivo and in vitro. The results revealed that TRPV2 expression was reduced in the cortex and hippocampus of AD model mice and AD patients. Cannabidiol enhanced microglial amyloid-β phagocytosis through TRPV2 activation, which increased the mRNA expression of the phagocytosis-related receptors, but knockdown of TRPV2 or Trem2 rescued the expression. TRPV2-mediated effects were also dependent on PDK1/Akt signaling, a pathway in which autophagy was indispensable. Furthermore, cannabidiol treatment successfully attenuated neuroinflammation while simultaneously improving mitochondrial function and ATP production via TRPV2 activation. Therefore, TRPV2 is proposed as a potential therapeutic target in AD, while CBD is a promising drug candidate for AD.
Keywords: Aβ1-42; TRPV2; autophagy; cannabidiol; phagocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tyrosine phosphorylation and palmitoylation of TRPV2 ion channel tune microglial beta-amyloid peptide phagocytosis.J Neuroinflammation. 2024 Sep 3;21(1):218. doi: 10.1186/s12974-024-03204-6. J Neuroinflammation. 2024. PMID: 39227967 Free PMC article.
-
Facilitating microglial phagocytosis by which Jiawei Xionggui Decoction alleviates cognitive impairment via TREM2-mediated energy metabolic reprogramming.Chin J Nat Med. 2025 Aug;23(8):909-919. doi: 10.1016/S1875-5364(25)60927-7. Chin J Nat Med. 2025. PMID: 40754372
-
Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation.Br J Pharmacol. 2014 May;171(9):2426-39. doi: 10.1111/bph.12615. Br J Pharmacol. 2014. PMID: 24641282 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
New insights in drug development for Alzheimer's disease based on microglia function.Biomed Pharmacother. 2021 Aug;140:111703. doi: 10.1016/j.biopha.2021.111703. Epub 2021 Jun 1. Biomed Pharmacother. 2021. PMID: 34083109 Review.
Cited by
-
Enhancing axonal myelination in seniors: A review exploring the potential impact cannabis has on myelination in the aged brain.Front Aging Neurosci. 2023 Mar 22;15:1119552. doi: 10.3389/fnagi.2023.1119552. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37032821 Free PMC article. Review.
-
A systematic study of molecular targets of cannabidiol in Alzheimer's disease.J Alzheimers Dis Rep. 2024 Oct 11;8(1):1339-1360. doi: 10.1177/25424823241284464. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 40034365 Free PMC article. Review.
-
The Protective Effect of CBD in a Model of In Vitro Ischemia May Be Mediated by Agonism on TRPV2 Channel and Microglia Activation.Int J Mol Sci. 2022 Oct 12;23(20):12144. doi: 10.3390/ijms232012144. Int J Mol Sci. 2022. PMID: 36292998 Free PMC article.
-
Cannabinerol (CBNR) Influences Synaptic Genes Associated with Cytoskeleton and Ion Channels in NSC-34 Cell Line: A Transcriptomic Study.Biomedicines. 2024 Jan 15;12(1):189. doi: 10.3390/biomedicines12010189. Biomedicines. 2024. PMID: 38255294 Free PMC article.
-
Effects of Cannabidiol on Innate Immunity: Experimental Evidence and Clinical Relevance.Int J Mol Sci. 2023 Feb 4;24(4):3125. doi: 10.3390/ijms24043125. Int J Mol Sci. 2023. PMID: 36834537 Free PMC article. Review.
References
-
- Graeber M.B., Kosel S., Egensperger R., Banati R.B., Muller U., Bise K., Hoff P., Moller H.J., Fujisawa K., Mehraein P. Rediscovery of the case described by Alois Alzheimer in 1911: Historical, histological and molecular genetic analysis. Neurogenetics. 1997;1:73–80. doi: 10.1007/s100480050011. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

