The Aryl Hydrocarbon Receptor Ligand FICZ Improves Left Ventricular Remodeling and Cardiac Function at the Onset of Pressure Overload-Induced Heart Failure in Mice
- PMID: 35628213
- PMCID: PMC9141655
- DOI: 10.3390/ijms23105403
The Aryl Hydrocarbon Receptor Ligand FICZ Improves Left Ventricular Remodeling and Cardiac Function at the Onset of Pressure Overload-Induced Heart Failure in Mice
Abstract
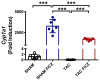
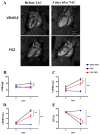
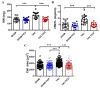
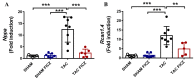
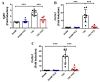
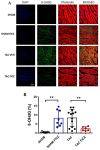
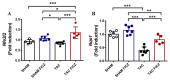
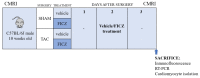
Adverse ventricular remodeling is the heart's response to damaging stimuli and is linked to heart failure and poor prognosis. Formyl-indolo [3,2-b] carbazole (FICZ) is an endogenous ligand for the aryl hydrocarbon receptor (AhR), through which it exerts pleiotropic effects including protection against inflammation, fibrosis, and oxidative stress. We evaluated the effect of AhR activation by FICZ on the adverse ventricular remodeling that occurs in the early phase of pressure overload in the murine heart induced by transverse aortic constriction (TAC). Cardiac structure and function were evaluated by cardiac magnetic resonance imaging (CMRI) before and 3 days after Sham or TAC surgery in mice treated with FICZ or with vehicle, and cardiac tissue was used for biochemical studies. CMRI analysis revealed that FICZ improved cardiac function and attenuated cardiac hypertrophy. These beneficial effects involved the inhibition of the hypertrophic calcineurin/NFAT pathway, transcriptional reduction in pro-fibrotic genes, and antioxidant effects mediated by the NRF2/NQO1 pathway. Overall, our findings provide new insight into the role of cardiac AhR signaling in the injured heart.
Keywords: aryl hydrocarbon receptor (AhR); cardiac hypertrophy; cardiac remodeling; fibrosis; formyl-indolo [3,2-b] carbazole (FICZ); oxidative stress.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
References
-
- Cohn J.N., Ferrari R., Sharpe N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000;35:569–582. doi: 10.1016/S0735-1097(99)00630-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- SAF2017-84777-R/Ministry of Economy, Industry and Competitiveness
- PID2020-113238RB-I00/Ministry of Science and Innovation (MCIN)/AEI/ 10.13039/501100011033 of Spain and the "European Union Next GenerationEU/PRTR
- PI20/01482-1/Instituto de Salud Carlos III
- CB16/11/00222/Centro de Investigación Biomédica en Red en Enfermedades Cardiovasculares
- Proyectos 2021/Universidad Francisco de Vitoria
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

