Cellulose-Based Nanomaterials Advance Biomedicine: A Review
- PMID: 35628218
- PMCID: PMC9140895
- DOI: 10.3390/ijms23105405
Cellulose-Based Nanomaterials Advance Biomedicine: A Review
Abstract
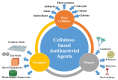
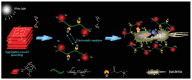
There are various biomaterials, but none fulfills all requirements. Cellulose biopolymers have advanced biomedicine to satisfy high market demand and circumvent many ecological concerns. This review aims to present an overview of cellulose knowledge and technical biomedical applications such as antibacterial agents, antifouling, wound healing, drug delivery, tissue engineering, and bone regeneration. It includes an extensive bibliography of recent research findings from fundamental and applied investigations. Cellulose-based materials are tailorable to obtain suitable chemical, mechanical, and physical properties required for biomedical applications. The chemical structure of cellulose allows modifications and simple conjugation with several materials, including nanoparticles, without tedious efforts. They render the applications cheap, biocompatible, biodegradable, and easy to shape and process.
Keywords: antibacterial; biomedical; cellulose; drug delivery; tissue engineering; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Nikzamir M., Akbarzadeh A., Panahi Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2021;61:102316. doi: 10.1016/j.jddst.2020.102316. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

