Mechanisms of the FMR1 Repeat Instability: How Does the CGG Sequence Expand?
- PMID: 35628235
- PMCID: PMC9141726
- DOI: 10.3390/ijms23105425
Mechanisms of the FMR1 Repeat Instability: How Does the CGG Sequence Expand?
Abstract
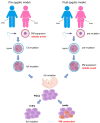
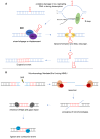
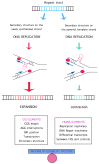
A dynamic mutation in exon 1 of the FMR1 gene causes Fragile X-related Disorders (FXDs), due to the expansion of an unstable CGG repeat sequence. Based on the CGG sequence size, two types of FMR1 alleles are possible: “premutation” (PM, with 56-200 CGGs) and “full mutation” (FM, with >200 triplets). Premutated females are at risk of transmitting a FM allele that, when methylated, epigenetically silences FMR1 and causes Fragile X syndrome (FXS), a very common form of inherited intellectual disability (ID). Expansions events of the CGG sequence are predominant over contractions and are responsible for meiotic and mitotic instability. The CGG repeat usually includes one or more AGG interspersed triplets that influence allele stability and the risk of transmitting FM to children through maternal meiosis. A unique mechanism responsible for repeat instability has not been identified, but several processes are under investigations using cellular and animal models. The formation of unusual secondary DNA structures at the expanded repeats are likely to occur and contribute to the CGG expansion. This review will focus on the current knowledge about CGG repeat instability addressing the CGG sequence expands.
Keywords: CGG repeat; FMR1 gene; dynamic mutations; mechanisms of instability; molecular medicine; neurological disease; repeat expansion disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Verkerk A.J., Pieretti M., Sutcliffe J.S., Fu Y.-H., Kuhl D.P., Pizzuti A., Reiner O., Richards S., Victoria M.F., Zhang F., et al. Identification of a gene (FMR1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-H. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

