Fasciola hepatica Gastrodermal Cells Selectively Release Extracellular Vesicles via a Novel Atypical Secretory Mechanism
- PMID: 35628335
- PMCID: PMC9143473
- DOI: 10.3390/ijms23105525
Fasciola hepatica Gastrodermal Cells Selectively Release Extracellular Vesicles via a Novel Atypical Secretory Mechanism
Abstract
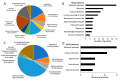
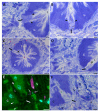
The liver fluke, Fasciola hepatica, is an obligate blood-feeder, and the gastrodermal cells of the parasite form the interface with the host's blood. Despite their importance in the host-parasite interaction, in-depth proteomic analysis of the gastrodermal cells is lacking. Here, we used laser microdissection of F. hepatica tissue sections to generate unique and biologically exclusive tissue fractions of the gastrodermal cells and tegument for analysis by mass spectrometry. A total of 226 gastrodermal cell proteins were identified, with proteases that degrade haemoglobin being the most abundant. Other detected proteins included those such as proton pumps and anticoagulants which maintain a microenvironment that facilitates digestion. By comparing the gastrodermal cell proteome and the 102 proteins identified in the laser microdissected tegument with previously published tegument proteomic datasets, we showed that one-quarter of proteins (removed by freeze-thaw extraction) or one-third of proteins (removed by detergent extraction) previously identified as tegumental were instead derived from the gastrodermal cells. Comparative analysis of the laser microdissected gastrodermal cells, tegument, and F. hepatica secretome revealed that the gastrodermal cells are the principal source of secreted proteins, as well as showed that both the gastrodermal cells and the tegument are likely to release subpopulations of extracellular vesicles (EVs). Microscopical examination of the gut caeca from flukes fixed immediately after their removal from the host bile ducts showed that selected gastrodermal cells underwent a progressive thinning of the apical plasma membrane which ruptured to release secretory vesicles en masse into the gut lumen. Our findings suggest that gut-derived EVs are released via a novel atypical secretory route and highlight the importance of the gastrodermal cells in nutrient acquisition and possible immunomodulation by the parasite.
Keywords: Fasciola hepatica; extracellular vesicle; helminth; proteome; secretion; trematode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Andrews S.J. The Life Cycle of Fasciola hepatica. In: Dalton J.P., editor. Fascioliasis. CABI; Oxford, UK: 1999. pp. 1–29.
-
- Fairweather I., Threadgold L.T., Hanna R.E.B. Development of Fasciola hepatica in the Mammalian Host. In: Dalton J.P., editor. Fascioliasis. CABI; Oxford, UK: 1999. pp. 47–103.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

