Adsorption of Biomineralization Protein Mms6 on Magnetite (Fe3O4) Nanoparticles
- PMID: 35628364
- PMCID: PMC9143127
- DOI: 10.3390/ijms23105554
Adsorption of Biomineralization Protein Mms6 on Magnetite (Fe3O4) Nanoparticles
Abstract
Biomineralization is an elaborate process that controls the deposition of inorganic materials in living organisms with the aid of associated proteins. Magnetotactic bacteria mineralize magnetite (Fe3O4) nanoparticles with finely tuned morphologies in their cells. Mms6, a magnetosome membrane specific (Mms) protein isolated from the surfaces of bacterial magnetite nanoparticles, plays an important role in regulating the magnetite crystal morphology. Although the binding ability of Mms6 to magnetite nanoparticles has been speculated, the interactions between Mms6 and magnetite crystals have not been elucidated thus far. Here, we show a direct adsorption ability of Mms6 on magnetite nanoparticles in vitro. An adsorption isotherm indicates that Mms6 has a high adsorption affinity (Kd = 9.52 µM) to magnetite nanoparticles. In addition, Mms6 also demonstrated adsorption on other inorganic nanoparticles such as titanium oxide, zinc oxide, and hydroxyapatite. Therefore, Mms6 can potentially be utilized for the bioconjugation of functional proteins to inorganic material surfaces to modulate inorganic nanoparticles for biomedical and medicinal applications.
Keywords: biomineralization; magnetotactic bacteria; metal oxide; nanoparticle; protein adsorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

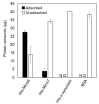
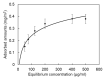



Similar articles
-
Intrinsic and extrinsic determinants of conditional localization of Mms6 to magnetosome organelles in Magnetospirillum magneticum AMB-1.J Bacteriol. 2024 Jun 20;206(6):e0000824. doi: 10.1128/jb.00008-24. Epub 2024 May 31. J Bacteriol. 2024. PMID: 38819153 Free PMC article.
-
Core Amino Acid Residues in the Morphology-Regulating Protein, Mms6, for Intracellular Magnetite Biomineralization.Sci Rep. 2016 Oct 19;6:35670. doi: 10.1038/srep35670. Sci Rep. 2016. PMID: 27759096 Free PMC article.
-
MMS6 protein regulates crystal morphology during nano-sized magnetite biomineralization in vivo.J Biol Chem. 2011 Feb 25;286(8):6386-92. doi: 10.1074/jbc.M110.183434. Epub 2010 Dec 18. J Biol Chem. 2011. PMID: 21169637 Free PMC article.
-
Crystallizing the function of the magnetosome membrane mineralization protein Mms6.Biochem Soc Trans. 2016 Jun 15;44(3):883-90. doi: 10.1042/BST20160057. Biochem Soc Trans. 2016. PMID: 27284056 Free PMC article. Review.
-
Learning from magnetotactic bacteria: A review on the synthesis of biomimetic nanoparticles mediated by magnetosome-associated proteins.J Struct Biol. 2016 Nov;196(2):75-84. doi: 10.1016/j.jsb.2016.06.026. Epub 2016 Jul 1. J Struct Biol. 2016. PMID: 27378728 Review.
Cited by
-
Iron acquisition and mineral transformation by cyanobacteria living in extreme environments.Mater Today Bio. 2022 Nov 15;17:100493. doi: 10.1016/j.mtbio.2022.100493. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36438421 Free PMC article.
-
Intrinsic and extrinsic determinants of conditional localization of Mms6 to magnetosome organelles in Magnetospirillum magneticum AMB-1.J Bacteriol. 2024 Jun 20;206(6):e0000824. doi: 10.1128/jb.00008-24. Epub 2024 May 31. J Bacteriol. 2024. PMID: 38819153 Free PMC article.
-
Magnetosomes as Potential Nanocarriers for Cancer Treatment.Curr Drug Deliv. 2024;21(8):1073-1081. doi: 10.2174/1567201820666230619155528. Curr Drug Deliv. 2024. PMID: 37340750 Review.
-
Bacterial imaging in tumour diagnosis.Microb Biotechnol. 2024 Jun;17(6):e14474. doi: 10.1111/1751-7915.14474. Microb Biotechnol. 2024. PMID: 38808743 Free PMC article. Review.
References
-
- Aizenberg J., Black A.J., Whitesides G.M. Control of crystal nucleation by patterned self-assembled monolayers. Nature. 1999;398:495–498. doi: 10.1038/19047. - DOI
-
- Weaver J.C., Wang Q., Miserez A., Tantuccio A., Stromberg R., Bozhilov K.N., Maxwell P., Nay R., Heier S.T., DiMasi E., et al. Analysis of an ultra hard magnetic biomineral in chiton radular teeth. Mater. Today. 2010;13:42–52. doi: 10.1016/S1369-7021(10)70016-X. - DOI

