Ancient Origins of Cytoskeletal Crosstalk: Spectraplakin-like Proteins Precede the Emergence of Cortical Microtubule Stabilization Complexes as Crosslinkers
- PMID: 35628404
- PMCID: PMC9145010
- DOI: 10.3390/ijms23105594
Ancient Origins of Cytoskeletal Crosstalk: Spectraplakin-like Proteins Precede the Emergence of Cortical Microtubule Stabilization Complexes as Crosslinkers
Abstract
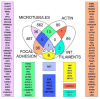
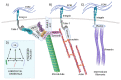
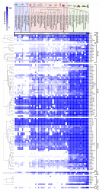
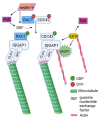
Adhesion between cells and the extracellular matrix (ECM) is one of the prerequisites for multicellularity, motility, and tissue specialization. Focal adhesions (FAs) are defined as protein complexes that mediate signals from the ECM to major components of the cytoskeleton (microtubules, actin, and intermediate filaments), and their mutual communication determines a variety of cellular processes. In this study, human cytoskeletal crosstalk proteins were identified by comparing datasets with experimentally determined cytoskeletal proteins. The spectraplakin dystonin was the only protein found in all datasets. Other proteins (FAK, RAC1, septin 9, MISP, and ezrin) were detected at the intersections of FAs, microtubules, and actin cytoskeleton. Homology searches for human crosstalk proteins as queries were performed against a predefined dataset of proteomes. This analysis highlighted the importance of FA communication with the actin and microtubule cytoskeleton, as these crosstalk proteins exhibit the highest degree of evolutionary conservation. Finally, phylogenetic analyses elucidated the early evolutionary history of spectraplakins and cortical microtubule stabilization complexes (CMSCs) as model representatives of the human cytoskeletal crosstalk. While spectraplakins probably arose at the onset of opisthokont evolution, the crosstalk between FAs and microtubules is associated with the emergence of metazoans. The multiprotein complexes contributing to cytoskeletal crosstalk in animals gradually gained in complexity from the onset of metazoan evolution.
Keywords: actin; cortical microtubule stabilization complex; cytoskeletal crosstalk; dystonin; evolution; focal adhesion; intermediate filaments; microtubule; spectraplakin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network.Mol Biol Cell. 2010 May 15;21(10):1714-24. doi: 10.1091/mbc.e10-01-0011. Epub 2010 Mar 24. Mol Biol Cell. 2010. PMID: 20335501 Free PMC article.
-
Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules.IUBMB Life. 2015 Jun;67(6):395-403. doi: 10.1002/iub.1384. Epub 2015 Jun 24. IUBMB Life. 2015. PMID: 26104829 Review.
-
Spectraplakin family proteins - cytoskeletal crosslinkers with versatile roles.J Cell Sci. 2017 Aug 1;130(15):2447-2457. doi: 10.1242/jcs.196154. Epub 2017 Jul 5. J Cell Sci. 2017. PMID: 28679697 Free PMC article. Review.
-
Spectraplakins: the cytoskeleton's Swiss army knife.Cell. 2008 Oct 3;135(1):16-8. doi: 10.1016/j.cell.2008.09.023. Cell. 2008. PMID: 18854149
-
ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity.Cell. 2008 Oct 3;135(1):137-48. doi: 10.1016/j.cell.2008.07.045. Cell. 2008. PMID: 18854161 Free PMC article.
Cited by
-
TRPML1 ion channel promotes HepaRG cell differentiation under simulated microgravity conditions.NPJ Microgravity. 2025 Mar 15;11(1):9. doi: 10.1038/s41526-025-00461-4. NPJ Microgravity. 2025. PMID: 40089547 Free PMC article.
-
Septins as membrane influencers: direct play or in association with other cytoskeleton partners.Front Cell Dev Biol. 2023 Feb 17;11:1112319. doi: 10.3389/fcell.2023.1112319. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36875762 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

