The Involvement of Alarmins in the Pathogenesis of Sjögren's Syndrome
- PMID: 35628481
- PMCID: PMC9145074
- DOI: 10.3390/ijms23105671
The Involvement of Alarmins in the Pathogenesis of Sjögren's Syndrome
Abstract
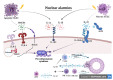
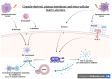
Sjögren's syndrome (SS) is a chronic autoimmune disease that affects exocrine glands, primarily the salivary and lachrymal glands. It is characterized by lymphoplasmacytic infiltration of the glandular tissues, ultimately leading to their dysfunction and destruction. Besides classic dry eyes and dry mouth defined as sicca syndrome, patients affected by the disease also typically display symptoms such as fatigue, pain and in more than 50% of cases, systemic manifestations such as arthritis, interstitial lung involvement, neurological involvement and an increased risk of lymphoma. The pathophysiological mechanisms underlying SS still remain elusive. The crucial role of innate immunity has been advocated in recent years regarding the pathogenesis of pSS, especially in the initiation and progression toward autoimmunity. Alarmins are endogenous molecules that belong to the large family of damage associated molecular pattern (DAMP). Alarmins are rapidly released, ensuing cell injury and interacting with pattern recognition receptors (PRR) such as toll-like receptors (TLR) to recruit and activate cells of the innate immune system and to promote adaptive immunity responses. This review highlights the current knowledge of various alarmins and their role in the pathogenesis of pSS.
Keywords: Sjögren’s syndrome; alarmins; autoimmunity; cytokines; damage associated molecular pattern; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fox R.I., Michelson P. Approaches to the treatment of Sjögren’s syndrome. J. Rheumatol. Suppl. 2000;61:15–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

