Vitamin K-Dependent Protein Activation: Normal Gamma-Glutamyl Carboxylation and Disruption in Disease
- PMID: 35628569
- PMCID: PMC9146348
- DOI: 10.3390/ijms23105759
Vitamin K-Dependent Protein Activation: Normal Gamma-Glutamyl Carboxylation and Disruption in Disease
Abstract
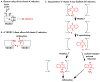
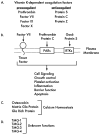
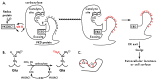
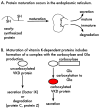
Vitamin K-dependent (VKD) proteins undergo an unusual post-translational modification, which is the conversion of specific Glu residues to carboxylated Glu (Gla). Gla generation is required for the activation of VKD proteins, and occurs in the endoplasmic reticulum during their secretion to either the cell surface or from the cell. The gamma-glutamyl carboxylase produces Gla using reduced vitamin K, which becomes oxygenated to vitamin K epoxide. Reduced vitamin K is then regenerated by a vitamin K oxidoreductase (VKORC1), and this interconversion of oxygenated and reduced vitamin K is referred to as the vitamin K cycle. Many of the VKD proteins support hemostasis, which is suppressed during therapy with warfarin that inhibits VKORC1 activity. VKD proteins also impact a broad range of physiologies beyond hemostasis, which includes regulation of calcification, apoptosis, complement, growth control, signal transduction and angiogenesis. The review covers the roles of VKD proteins, how they become activated, and how disruption of carboxylation can lead to disease. VKD proteins contain clusters of Gla residues that form a calcium-binding module important for activity, and carboxylase processivity allows the generation of multiple Glas. The review discusses how impaired carboxylase processivity results in the pseudoxanthoma elasticum-like disease.
Keywords: VKCFD; gamma-glutamyl carboxylase (GGCX); processivity; pseudoxanthoma elasticum-like (PXE-like); vitamin K; vitamin K oxidoreductase (VKORC1); vitamin K-dependent proteins; warfarin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

