Towards 3D Bioprinted Spinal Cord Organoids
- PMID: 35628601
- PMCID: PMC9144715
- DOI: 10.3390/ijms23105788
Towards 3D Bioprinted Spinal Cord Organoids
Abstract
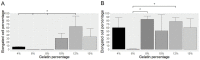
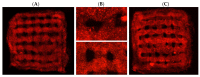
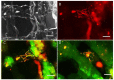
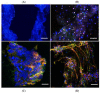
Three-dimensional (3D) cultures, so-called organoids, have emerged as an attractive tool for disease modeling and therapeutic innovations. Here, we aim to determine if boundary cap neural crest stem cells (BC) can survive and differentiate in gelatin-based 3D bioprinted bioink scaffolds in order to establish an enabling technology for the fabrication of spinal cord organoids on a chip. BC previously demonstrated the ability to support survival and differentiation of co-implanted or co-cultured cells and supported motor neuron survival in excitotoxically challenged spinal cord slice cultures. We tested different combinations of bioink and cross-linked material, analyzed the survival of BC on the surface and inside the scaffolds, and then tested if human iPSC-derived neural cells (motor neuron precursors and astrocytes) can be printed with the same protocol, which was developed for BC. We showed that this protocol is applicable for human cells. Neural differentiation was more prominent in the peripheral compared to central parts of the printed construct, presumably because of easier access to differentiation-promoting factors in the medium. These findings show that the gelatin-based and enzymatically cross-linked hydrogel is a suitable bioink for building a multicellular, bioprinted spinal cord organoid, but that further measures are still required to achieve uniform neural differentiation.
Keywords: bioprinting; cell differentiation; cell survival; hydrogel; neural stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

