Development and Characterization of a Factor V-Deficient CRISPR Cell Model for the Correction of Mutations
- PMID: 35628611
- PMCID: PMC9148015
- DOI: 10.3390/ijms23105802
Development and Characterization of a Factor V-Deficient CRISPR Cell Model for the Correction of Mutations
Abstract
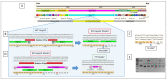
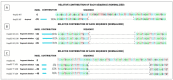
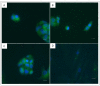
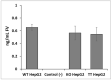
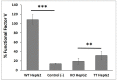
Factor V deficiency, an ultra-rare congenital coagulopathy, is characterized by bleeding episodes that may be more or less intense as a function of the levels of coagulation factor activity present in plasma. Fresh-frozen plasma, often used to treat patients with factor V deficiency, is a scarcely effective palliative therapy with no specificity to the disease. CRISPR/Cas9-mediated gene editing, following precise deletion by non-homologous end-joining, has proven to be highly effective for modeling on a HepG2 cell line a mutation similar to the one detected in the factor V-deficient patient analyzed in this study, thus simulating the pathological phenotype. Additional CRISPR/Cas9-driven non-homologous end-joining precision deletion steps allowed correction of 41% of the factor V gene mutated cells, giving rise to a newly developed functional protein. Taking into account the plasma concentrations corresponding to the different levels of severity of factor V deficiency, it may be argued that the correction achieved in this study could, in ideal conditions, be sufficient to turn a severe phenotype into a mild or asymptomatic one.
Keywords: CRISPR; coagulopathies; factor V deficiency; gene editing; gene therapy; rare diseases.
Conflict of interest statement
The authors have declared to have no conflict of interest with respect to this study.
Figures


Similar articles
-
Successful correction of factor V deficiency of patient-derived iPSCs by CRISPR/Cas9-mediated gene editing.Haemophilia. 2020 Sep;26(5):826-833. doi: 10.1111/hae.14104. Epub 2020 Jul 22. Haemophilia. 2020. PMID: 32700411
-
Development of a novel and viable knock-in factor V deficiency murine model: Utility for an ultra-rare disease.PLoS One. 2025 Jun 2;20(6):e0321864. doi: 10.1371/journal.pone.0321864. eCollection 2025. PLoS One. 2025. PMID: 40455764 Free PMC article.
-
The most common disease-causing mutation of factor XIII deficiency is corrected by CRISPR/CAS9 gene editing system.Blood Coagul Fibrinolysis. 2022 Apr 1;33(3):153-158. doi: 10.1097/MBC.0000000000001126. Epub 2022 Feb 25. Blood Coagul Fibrinolysis. 2022. PMID: 35221320
-
A Comprehensive Overview of Coagulation Factor V and Congenital Factor V Deficiency.Semin Thromb Hemost. 2019 Jul;45(5):523-543. doi: 10.1055/s-0039-1687906. Epub 2019 May 23. Semin Thromb Hemost. 2019. PMID: 31121608 Review.
-
CRISPR/Cas9-mediated correction of human genetic disease.Sci China Life Sci. 2017 May;60(5):447-457. doi: 10.1007/s11427-017-9032-4. Epub 2017 May 3. Sci China Life Sci. 2017. PMID: 28534256 Review.
Cited by
-
CRISPR/Cas9-Mediated Generation of COL7A1-Deficient Keratinocyte Model of Recessive Dystrophic Epidermolysis Bullosa.Cell J. 2023 Oct 9;25(10):665-673. doi: 10.22074/cellj.2023.1989321.1225. Cell J. 2023. PMID: 37865875 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

