Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis
- PMID: 35628698
- PMCID: PMC9146234
- DOI: 10.3390/jof8050442
Therapeutic Potential of Green Synthesized Gold Nanoparticles Using Extract of Leptadenia hastata against Invasive Pulmonary Aspergillosis
Abstract
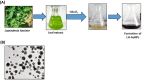
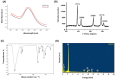
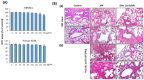
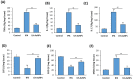
Gold nanoparticles are widely used in the biomedical field for the treatment of several diseases, including cancer, inflammatory diseases, and immune system disorders, due to their distinctive physicochemical characteristics. In this study, we investigated the therapeutic potential of green synthesized gold nanoparticles using ethanolic leaf extract of Leptadenia hastata (LH-AuNPs) against invasive pulmonary aspergillosis (IPA) in mice. UV/visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), and zeta potential were used to characterize the biofabricated LH-AuNPs. Antifungal activity of LH-AuNPs was determined by MTT assay, (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide), time-kill assay, and radial growth inhibition. TEM and SEM were used to examine the mode of the antifungal action of LH-AuNPs. The in vivo activity of LH-AuNPs against IPA was studied using a well-established IPA mouse model. LH-AuNPs excreted antifungal activity against Aspergillus fumigatus with MIC 64 µg/mL and inhibited the radial growth of A. fumigatus by 30% compared to the control. LH-AuNPs caused distortion and collapse of fungal hyphae and deterioration of cell walls. Interestingly, LH-AuNPs did not display any cytotoxicity on cultured primary bone marrow stem cells (BMSCs) or A549 human lung cell line in vitro at MIC concentration. IPA mice treated with LH-AuNPs displayed significant lung tissue repair without any in vivo cytotoxicity. LH-AuNPs administration showed significant suppression of fungal burden and gliotoxin production in the lung. In addition, LH-AuNPs inhibited IPA-induced pro-inflammatory cytokines production, including interleukin-1 (IL-1), interleukin-17 (IL-17), and tumor necrosis factor-alpha (TNF-α), and reduced oxidative stress in lung. In conclusion, our data provide LH-AuNPs as a novel nanoparticle therapy for IPA.
Keywords: A. fumigatus; IPA; Leptadenia hastata; aspergillosis; gold nanoparticles.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures



References
-
- Zarrinfar H., Mirhendi H., Fata A., Khodadadi H., Kordbacheh P. Detection of Aspergillus flavus and A. fumigatus in Bronchoalveolar Lavage Specimens of Hematopoietic Stem Cell Transplants and Hematological Malignancies Patients by Real-Time Polymerase Chain Reaction, Nested PCR and Mycological Assays. Jundishapur J. Microbiol. 2015;8:e13744. - PMC - PubMed
-
- Kashefi E., Seyedi S.J., Zomorodian K., Zare Shahrabadi Z., Zarrinfar H. Successful treatment of pulmonary aspergillosis due to Aspergillus fumigatus in a child affected by systemic lupus erythematosus: A case report from Northeastern Iran. Clin. Case Rep. 2021;9:e04248. doi: 10.1002/ccr3.4248. - DOI - PMC - PubMed
-
- Groll A.H., Piscitelli S.C., Walsh T.J. Clinical pharmacology of systemic antifungal agents: A comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 1998;44:343–500. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

