Design and Rationale of the Sevoflurane for Sedation in Acute Respiratory Distress Syndrome (SESAR) Randomized Controlled Trial
- PMID: 35628922
- PMCID: PMC9147018
- DOI: 10.3390/jcm11102796
Design and Rationale of the Sevoflurane for Sedation in Acute Respiratory Distress Syndrome (SESAR) Randomized Controlled Trial
Abstract
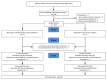
Preclinical studies have shown that volatile anesthetics may have beneficial effects on injured lungs, and pilot clinical data support improved arterial oxygenation, attenuated inflammation, and decreased lung epithelial injury in patients with acute respiratory distress syndrome (ARDS) receiving inhaled sevoflurane compared to intravenous midazolam. Whether sevoflurane is effective in improving clinical outcomes among patients with ARDS is unknown, and the benefits and risks of inhaled sedation in ARDS require further evaluation. Here, we describe the SESAR (Sevoflurane for Sedation in ARDS) trial designed to address this question. SESAR is a two-arm, investigator-initiated, multicenter, prospective, randomized, stratified, parallel-group clinical trial with blinded outcome assessment designed to test the efficacy of sedation with sevoflurane compared to intravenous propofol in patients with moderate to severe ARDS. The primary outcome is the number of days alive and off the ventilator at 28 days, considering death as a competing event, and the key secondary outcome is 90 day survival. The planned enrollment is 700 adult participants at 37 French academic and non-academic centers. Safety and long-term outcomes will be evaluated, and biomarker measurements will help better understand mechanisms of action. The trial is funded by the French Ministry of Health, the European Society of Anaesthesiology, and Sedana Medical.
Keywords: acute respiratory distress syndrome; clinical trial; inhaled sedation; sevoflurane.
Conflict of interest statement
M.J. is a principal investigator of the ISCA study (ClinicalTrials.gov Identifier: NCT04383730), with grants from Sedana Medical; J.-M.C. and M.J. received fees from Sedana Medical for participation in a scientific advisory panel in 2019; M.J. received fees from Sedana Medical for participation in seminars in 2019, 2020, and 2021; M.J. received consulting fees in 2020 and 2021, and fees for participation in a scientific advisory panel in 2021, from Abbvie. Neither Sedana Medical nor Abbvie have any influence on the study design, collection, analysis, interpretation of data, and writing. The other authors have nothing to disclose. All authors have read and agreed to the published version of the manuscript.
Figures

References
-
- DAS-Taskforce 2015. Baron R., Binder A., Biniek R., Braune S., Buerkle H., Dall P., Demirakca S., Eckardt R., Eggers V., et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015)-short version. Ger. Med. Sci. 2015;13:19. - PMC - PubMed
-
- Blondonnet R., Quinson A., Lambert C., Audard J., Godet T., Zhai R., Pereira B., Futier E., Bazin J.-E., Constantin J.-M., et al. Use of Volatile Agents for Sedation in the Intensive Care Unit: A National Survey in France. PLoS ONE. 2021;16:e0249889. doi: 10.1371/journal.pone.0249889. - DOI - PMC - PubMed
-
- Meiser A., Volk T., Wallenborn J., Guenther U., Becher T., Bracht H., Schwarzkopf K., Knafelj R., Faltlhauser A., Thal S.C., et al. Inhaled Isoflurane via the Anaesthetic Conserving Device versus Propofol for Sedation of Invasively Ventilated Patients in Intensive Care Units in Germany and Slovenia: An Open-Label, Phase 3, Randomised Controlled, Non-Inferiority Trial. Lancet Respir. Med. 2021;9:1231–1240. doi: 10.1016/S2213-2600(21)00323-4. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

