Blood Transfusion Reactions-A Comprehensive Review of the Literature including a Swiss Perspective
- PMID: 35628985
- PMCID: PMC9144124
- DOI: 10.3390/jcm11102859
Blood Transfusion Reactions-A Comprehensive Review of the Literature including a Swiss Perspective
Abstract
Blood transfusions have been the cornerstone of life support since the introduction of the ABO classification in the 20th century. The physiologic goal is to restore adequate tissue oxygenation when the demand exceeds the offer. Although it can be a life-saving therapy, blood transfusions can lead to serious adverse effects, and it is essential that physicians remain up to date with the current literature and are aware of the pathophysiology, initial management and risks of each type of transfusion reaction. We aim to provide a structured overview of the pathophysiology, clinical presentation, diagnostic approach and management of acute transfusion reactions based on the literature available in 2022. The numbers of blood transfusions, transfusion reactions and the reporting rate of transfusion reactions differ between countries in Europe. The most frequent transfusion reactions in 2020 were alloimmunizations, febrile non-hemolytic transfusion reactions and allergic transfusion reactions. Transfusion-related acute lung injury, transfusion-associated circulatory overload and septic transfusion reactions were less frequent. Furthermore, the COVID-19 pandemic has challenged the healthcare system with decreasing blood donations and blood supplies, as well as rising concerns within the medical community but also in patients about blood safety and transfusion reactions in COVID-19 patients. The best way to prevent transfusion reactions is to avoid unnecessary blood transfusions and maintain a transfusion-restrictive strategy. Any symptom occurring within 24 h of a blood transfusion should be considered a transfusion reaction and referred to the hemovigilance reporting system. The initial management of blood transfusion reactions requires early identification, immediate interruption of the transfusion, early consultation of the hematologic and ICU departments and fluid resuscitation.
Keywords: adverse transfusion reactions; anemia treatment; blood cell transfusion; erythrocyte transfusion; pulmonary complications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

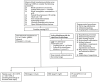
References
-
- Swissmedic Analyse des Annonces D’hémovigilance. 2020. [(accessed on 31 January 2022)]. Available online: https://www.swissmedic.ch/swissmedic/fr/home/humanarzneimittel/marktuebe....
-
- Swissmedic 2019 © Copyright The Swiss Haemovigilance Reporting System—Fundamentals. Swissmedic-Analyse des Annonces D’hémovigilance. 2018. [(accessed on 2 May 2022)]. Available online: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktuebe....
-
- Swissmedic 2019 © Copyright The Swiss Haemovigilance Reporting System—Fundamentals. Swissmedic-Analyse des Annonces D’hémovigilance. 2017. [(accessed on 2 May 2022)]. Available online: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktuebe....
-
- L’Agence Nationale de Sécurité du Médicament et des Produits de Santé-18eme Rapport National D’hémovigilance Dec, 2021. [(accessed on 23 March 2022)]. Available online: https://ansm.sante.fr/uploads/2021/12/08/20211208-rapport-hemovigilance-....
-
- Funk M.B., Heiden M., Muller S. Hämovigilanz-Bericht des Paul-Ehrlich-Instituts 2020: Auswertung der Meldungen von Reaktionen und Zwischenfällen nach § 63i AMG. 2021. [(accessed on 26 March 2022)]. Available online: www.pei.de/haemovigilanzbericht.
Publication types
LinkOut - more resources
Full Text Sources

