New Insights into the Pathogenesis of Giant Cell Arteritis: Mechanisms Involved in Maintaining Vascular Inflammation
- PMID: 35629030
- PMCID: PMC9143803
- DOI: 10.3390/jcm11102905
New Insights into the Pathogenesis of Giant Cell Arteritis: Mechanisms Involved in Maintaining Vascular Inflammation
Abstract
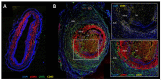
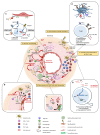
The giant cell arteritis (GCA) pathophysiology is complex and multifactorial, involving a predisposing genetic background, the role of immune aging and the activation of vascular dendritic cells by an unknown trigger. Once activated, dendritic cells recruit CD4 T cells and induce their activation, proliferation and polarization into Th1 and Th17, which produce interferon-gamma (IFN-γ) and interleukin-17 (IL-17), respectively. IFN-γ triggers the production of chemokines by vascular smooth muscle cells, which leads to the recruitment of additional CD4 and CD8 T cells and also monocytes that differentiate into macrophages. Recent data have shown that IL-17, IFN-γ and GM-CSF induce the differentiation of macrophage subpopulations, which play a role in the destruction of the arterial wall, in neoangiogenesis or intimal hyperplasia. Under the influence of different mediators, mainly endothelin-1 and PDGF, vascular smooth muscle cells migrate to the intima, proliferate and change their phenotype to become myofibroblasts that further proliferate and produce extracellular matrix proteins, increasing the vascular stenosis. In addition, several defects in the immune regulatory mechanisms probably contribute to chronic vascular inflammation in GCA: a defect in the PD-1/PD-L1 pathway, a quantitative and qualitative Treg deficiency, the implication of resident cells, the role of GM-CSF and IL-6, the implication of the NOTCH pathway and the role of mucosal-associated invariant T cells and tissue-resident memory T cells.
Keywords: GM-CSF; IL-6; T cells; giant cell arteritis; pathogenesis; vascular smooth muscle cells.
Conflict of interest statement
Maxime Samson: Abbvie (consultant), Boerhinger Ingelheim (consultant), Vifor Pharma (consultant and invitation to congresses), Roche Chugai (consultant and invitation to congresses), Novartis (consultant and research grant); Bernard Bonnotte: Roche-Chugai (consultant and remuneration for symposiums), Boerhinger Ingelheim (consultant).
Figures

References
-
- Espitia O., Samson M., Le Gallou T., Connault J., Landron C., Lavigne C., Belizna C., Magnant J., de Moreuil C., Roblot P., et al. Comparison of idiopathic (isolated) aortitis and giant cell arteritis-related aortitis. A French retrospective multicenter study of 117 patients. Autoimmun. Rev. 2016;15:571–576. doi: 10.1016/j.autrev.2016.02.016. - DOI - PubMed
-
- Salvarani C., Macchioni P., Zizzi F., Mantovani W., Rossi F., Castri C., Capozzoli N., Baricchi R., Boiardi L., Chiaravalloti F., et al. Epidemiologic and immunogenetic aspects of polymyalgia rheumatica and giant cell arteritis in northern Italy. Arthritis Rheum. 1991;34:351–356. doi: 10.1002/art.1780340313. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

