Impaired Humoral Immunity with Concomitant Preserved T Cell Reactivity in IBD Patients on Treatment with Infliximab 6 Month after Vaccination with the SARS-CoV-2 mRNA Vaccine BNT162b2: A Pilot Study
- PMID: 35629116
- PMCID: PMC9146879
- DOI: 10.3390/jpm12050694
Impaired Humoral Immunity with Concomitant Preserved T Cell Reactivity in IBD Patients on Treatment with Infliximab 6 Month after Vaccination with the SARS-CoV-2 mRNA Vaccine BNT162b2: A Pilot Study
Abstract
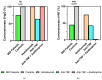
Introduction: The Coronavirus Disease 2019 (COVID-19) pandemic has been caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The most important approach to prevent severe disease progression and to contain the pandemic is the use of COVID-19 vaccines. The aim of this study was to investigate the humoral and cellular response in immunosuppressed patients with inflammatory bowel disease (IBD) on treatment with anti-TNF (infliximab, adalimumab) and anti-α4ß7-Integrin (vedolizumab) 6 months after mRNA vaccination against SARS-CoV-2 compared to healthy subjects. Methods: In this prospective study, 20 IBD patients and 9 healthy controls were included 6 months after the second BNT162b2 vaccination. In addition to quantitative determination of IgG antibody levels against the SARS-CoV-2 receptor-binding domain (RBD) of the spike protein subunit S1, a SARS-CoV-2 surrogate neutralization test (sVNT) was used to assess potential neutralization capacity. SARS-CoV-2-specific T-cell responses were measured using an interferon-γ (IFN-γ) release assay (IGRA; Euroimmun Medical Laboratory Diagnostics, Lübeck, Germany). Results: S-IgG could still be detected in the majority of IBD patients 6 months after second vaccination. Compared to healthy controls, IBD patients treated with anti-TNF agents showed both lower neutralizing activity in sVNT (percent inhibition of ACE2 receptor binding by RBD protein) and lower IgG-S (AU/mL) antibody levels (AB) (sVNT: 79% vs. 2%, p < 0. 001; AB: 1018 AU/mL vs. 141 AU/mL, p = 0.025). In contrast, patients on therapy with vedolizumab showed no impairment in humoral immune response (sVNT, S-IgG) compared with healthy controls. Specific T-cellular reactivity was detected in 73% of IBD patients and in 67% of healthy controls independent of immunosuppressive therapy (anti-TNF., vedolizumab) (p = 0.189). Conclusion: Six months after BNT162b2 vaccination, this study found significantly decreased antibody levels in patients under anti-TNF therapy. IBD patients under anti-TNF and vedolizumab therapy had no impairment of T-cellular reactivity compared to healthy controls at this time point. Further studies with larger collectives for confirmation should follow.
Keywords: COVID-19; IBD patients; SARS-CoV-2; humoral and T-cellular immune response; seroconversion; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. - DOI - PMC - PubMed
-
- Tepasse P.-R., Hafezi W., Lutz M., Kühn J., Wilms C., Wiewrodt R., Sackarnd J., Keller M., Schmidt H.H., Vollenberg R. Persisting SARS-CoV-2 viraemia after rituximab therapy: Two cases with fatal outcome and a review of the literature. Br. J. Haematol. 2020;190:185–188. doi: 10.1111/bjh.16896. - DOI - PMC - PubMed
-
- Kessel C., Vollenberg R., Masjosthusmann K., Hinze C., Wittkowski H., Debaugnies F., Nagant C., Corazza F., Vély F., Kaplanski G., et al. Discrimination of COVID-19 from Inflammation-Induced Cytokine Storm Syndromes Using Disease-Related Blood Biomarkers. Arthritis Rheumatol. 2021;73:1791–1799. doi: 10.1002/art.41763. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

