Optimization of Preoperative Lymph Node Staging in Patients with Muscle-Invasive Bladder Cancer Using Radiomics on Computed Tomography
- PMID: 35629148
- PMCID: PMC9147130
- DOI: 10.3390/jpm12050726
Optimization of Preoperative Lymph Node Staging in Patients with Muscle-Invasive Bladder Cancer Using Radiomics on Computed Tomography
Abstract
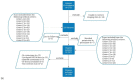
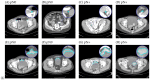
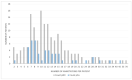
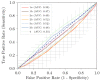
Approximately 25% of the patients with muscle-invasive bladder cancer (MIBC) who are clinically node negative have occult lymph node metastases at radical cystectomy (RC) and pelvic lymph node dissection. The aim of this study was to evaluate preoperative CT-based radiomics to differentiate between pN+ and pN0 disease in patients with clinical stage cT2-T4aN0-N1M0 MIBC. Patients with cT2-T4aN0-N1M0 MIBC, of whom preoperative CT scans and pathology reports were available, were included from the prospective, multicenter CirGuidance trial. After manual segmentation of the lymph nodes, 564 radiomics features were extracted. A combination of different machine-learning methods was used to develop various decision models to differentiate between patients with pN+ and pN0 disease. A total of 209 patients (159 pN0; 50 pN+) were included, with a total of 3153 segmented lymph nodes. None of the individual radiomics features showed significant differences between pN+ and pN0 disease, and none of the radiomics models performed substantially better than random guessing. Hence, CT-based radiomics does not contribute to differentiation between pN+ and pN0 disease in patients with cT2-T4aN0-N1M0 MIBC.
Keywords: bladder cancer; computed tomography; machine learning; radiomics.
Conflict of interest statement
A.C.M.v.d.L. receives speaker fees from Janssen and Eisa, and is an advisor to Janssen and Pfizer. W.J.N. is founder, scientific lead and stock-holder of Quantib BV. A.A.M.v.d.V. receives consulting honoraria from Sanofi, Roche, Merck Sharp & Dohme, Pfizer, Eisai, Ipsen, Novartis, Pierre Fabre and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this publication were reported.
Figures

Similar articles
-
Radiomics Signature Using Manual Versus Automated Segmentation for Lymph Node Staging of Bladder Cancer.Eur Urol Focus. 2023 Jan;9(1):145-153. doi: 10.1016/j.euf.2022.08.015. Epub 2022 Sep 14. Eur Urol Focus. 2023. PMID: 36115774 Clinical Trial.
-
Preoperative chemotherapy in clinically node positive muscle invasive bladder cancer: Radiologic variables can predict response.Urol Oncol. 2021 Feb;39(2):133.e1-133.e8. doi: 10.1016/j.urolonc.2020.08.020. Epub 2020 Sep 6. Urol Oncol. 2021. PMID: 32900621
-
Molecular lymph node staging for bladder cancer patients undergoing radical cystectomy with pelvic lymph node dissection.Urol Oncol. 2020 Jul;38(7):639.e11-639.e19. doi: 10.1016/j.urolonc.2020.01.018. Epub 2020 Mar 4. Urol Oncol. 2020. PMID: 32146127
-
[Pathological pelvic lymph node involvement in muscle-invasive bladder cancer patients treated with radical cystectomy: A narrative review].Prog Urol. 2023 Mar;33(3):145-154. doi: 10.1016/j.purol.2022.12.013. Epub 2023 Jan 4. Prog Urol. 2023. PMID: 36604248 Review. French.
-
Quality indicators for the management of muscle-invasive bladder cancer in the perioperative setting of radical cystectomy: a narrative review.Transl Cancer Res. 2022 Apr;11(4):908-917. doi: 10.21037/tcr-21-1116. Transl Cancer Res. 2022. PMID: 35571640 Free PMC article. Review.
Cited by
-
Unsupervised learning-based quantitative analysis of CT intratumoral subregions predicts risk stratification of bladder cancer patients.BMC Med. 2025 Jun 2;23(1):328. doi: 10.1186/s12916-025-04163-2. BMC Med. 2025. PMID: 40457401 Free PMC article.
-
Is CT Radiomics Superior to Morphological Evaluation for pN0 Characterization? A Pilot Study in Colon Cancer.Cancers (Basel). 2024 Feb 4;16(3):660. doi: 10.3390/cancers16030660. Cancers (Basel). 2024. PMID: 38339411 Free PMC article.
-
Radiomics vs radiologist in bladder and renal cancer. Results from a systematic review.Cent European J Urol. 2023;76(1):12-19. doi: 10.5173/ceju.2023.252. Epub 2023 Jan 21. Cent European J Urol. 2023. PMID: 37064257 Free PMC article. Review.
References
-
- Witjes J.A., Bruins H.M., Cathomas R., Compérat E.M., Cowan N.C., Gakis G., Hernández V., Linares Espinós E., Lorch A., Neuzillet Y., et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. - DOI - PubMed
-
- Stein J.P., Lieskovsky G., Raghavan D., Skinner D.G., Cote R., Groshen S., Feng A., Boyd S., Skinner E., Bochner B., et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. - DOI - PubMed
-
- Griffiths G., Hall R., Sylvester R., Raghavan D., Parmar M.K.B. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. J. Clin. Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. - DOI - PMC - PubMed
-
- Sternberg C.N., Yagoda A., Scher H.I., Watson R.C., Geller N., Herr H.W., Morse M.J., Sogani P.C., Vaughan E.D., Bander N., et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer. 1989;64:2448–2458. doi: 10.1002/1097-0142(19891215)64:12<2448::AID-CNCR2820641209>3.0.CO;2-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

