Targeting HIF-1α Function in Cancer through the Chaperone Action of NQO1: Implications of Genetic Diversity of NQO1
- PMID: 35629169
- PMCID: PMC9146583
- DOI: 10.3390/jpm12050747
Targeting HIF-1α Function in Cancer through the Chaperone Action of NQO1: Implications of Genetic Diversity of NQO1
Abstract
HIF-1α is a master regulator of oxygen homeostasis involved in different stages of cancer development. Thus, HIF-1α inhibition represents an interesting target for anti-cancer therapy. It was recently shown that the HIF-1α interaction with NQO1 inhibits proteasomal degradation of the former, thus suggesting that targeting the stability and/or function of NQO1 could lead to the destabilization of HIF-1α as a therapeutic approach. Since the molecular interactions of NQO1 with HIF-1α are beginning to be unraveled, in this review we discuss: (1) Structure-function relationships of HIF-1α; (2) our current knowledge on the intracellular functions and stability of NQO1; (3) the pharmacological modulation of NQO1 by small ligands regarding function and stability; (4) the potential effects of genetic variability of NQO1 in HIF-1α levels and function; (5) the molecular determinants of NQO1 as a chaperone of many different proteins including cancer-associated factors such as HIF-1α, p53 and p73α. This knowledge is then further discussed in the context of potentially targeting the intracellular stability of HIF-1α by acting on its chaperone, NQO1. This could result in novel anti-cancer therapies, always considering that the substantial genetic variability in NQO1 would likely result in different phenotypic responses among individuals.
Keywords: HIF-1α; NQO1; angiogenesis; cancer; genetic variability; hypoxia; ligand binding; proteasomal degradation; protein: protein interactions.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
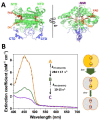
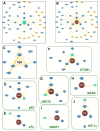
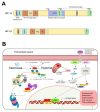




References
Publication types
Grants and funding
- RTI2018-096246-B-100/ERDF/Spanish Ministry of Science, Innovation and Universities-State Research Agency
- PID2019-110900GB-I00/ERDF/Spanish Ministry of Science, Innovation and Universities-State Research Agency
- SAF2015-69796/ERDF/Spanish Ministry of Science, Innovation and Universities-State Research Agency
- P18-RT-2413/Consejería de Economía, Conocimiento, Empresas y Universidad, Junta de Andalucía
- E35-20R/The Government of Aragón-FEDER.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

