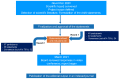
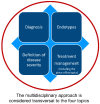
Personalized Management of Patients with Chronic Rhinosinusitis with Nasal Polyps in Clinical Practice: A Multidisciplinary Consensus Statement
- PMID: 35629268
- PMCID: PMC9143504
- DOI: 10.3390/jpm12050846
Personalized Management of Patients with Chronic Rhinosinusitis with Nasal Polyps in Clinical Practice: A Multidisciplinary Consensus Statement
Abstract
Chronic rhinosinusitis (CRS) is a sino-nasal chronic inflammatory disease, occurring in 5-15% of the general population. CRS with nasal polyps (CRSwNP) is present in up to 30% of the CRS population. One-third of CRSwNP patients suffer from disease that is uncontrolled by current standards of care. Biologics are an emerging treatment option for patients with severe uncontrolled CRSwNP, but their positioning in the treatment algorithm is under discussion. Effective endotyping of CRSwNP patients who could benefit from biologics treatment is required, as suggested by international guidelines. Other issues affecting management include comorbidities, such as allergy, non-steroidal anti-inflammatory drug-exacerbated respiratory disease, and asthma. Therefore, the choice of treatment in CRSwNP patients depends on many factors. A multidisciplinary approach may improve CRSwNP management in patients with comorbidities, but currently there is no shared management model. We summarize the outcomes of a Delphi process involving a multidisciplinary panel of otolaryngologists, pulmonologists, and allergist-immunologists involved in the management of CRSwNP, who attempted to reach consensus on key statements relating to the diagnosis, endotyping, classification and management (including the place of biologics) of CRSwNP patients.
Keywords: allergy; asthma; biologics; chronic rhinosinusitis; hypersensitivity; nasal polyps; non-steroidal anti-inflammatory drugs; type 2 inflammation.
Conflict of interest statement
EDC has received advisory board fees or speaker fees from GlaxoSmithKline, Novartis, and Sanofi-Genzyme. MBB has received advisory board fees or speaker fees from ALK-Abelló, AstraZeneca, GlaxoSmithKline, Menarini, Novartis, and Sanofi. VS has received speaker fees or advisory board fees from AstraZeneca, GlaxoSmithKline, Sanofi, and Novartis. FB has received research grants or advisory board fees or speaker fees from Menarini, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Guidotti-Malesci, GlaxoSmithKline, Merck Sharp & Dohme, Mundipharma, Novartis, Sanofi, Stallergenes-Greer, and Alfasigma. MG has received advisory board fees or speaker fees from GlaxoSmithKline, Novartis, Sanofi-Genzyme, Valeas, DMG Italia, FIRMA, Aurora Biofarma, and Fenix Pharma. EH has received speaker activity and advisory board participation fees from Novartis, GlaxoSmithKline, Sanofi, Regeneron, AstraZeneca, Stallergenes-Greer, Circassia, and Nestlè Purina. ML has received speaker fees or advisory board fees from GlaxoSmithKline, AstraZeneca, Chiesi Farmaceutici, Guidotti-Malesci, Sanofi, Menarini, and Alfasigma. GP has received lecture fees and advisory board fees from ABC, Alfasigma, AstraZeneca, GlaxoSmithKline, Guidotti-Malesci, Menarini, Novartis, Sanofi, and Zambon. GS has received advisory board fees or speaker fees from AstraZeneca, GlaxoSmithKline, Menarini, Novartis, and Sanofi. PC has, in the last two years, had received grants or fees from Karl Storz, Medtronic, Novartis, GlaxoSmithKline, and Sanofi-Aventis. GWC has received research grants, advisory board fees or speaker fees from Menarini, ALK-Abelló, Allergy Therapeutics, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, GlaxoSmithKline, Hal Allergy, Mylan, Merck Sharp & Dohme, Mundipharma, Novartis, Regeneron, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer, UCB Pharma, Valeas, and Vibor-Pharma. AM and LM have no relevant financial interests to disclose.
Figures
References
-
- Dietz de Loos D., Lourijsen E.S., Wildeman M.A.M., Freling N.J.M., Wolvers M.D.J., Reitsma S., Fokkens W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019;143:1207–1214. doi: 10.1016/j.jaci.2018.12.986. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources