Study on Preparation of Polymer-Modified Bentonite and Sand Mixtures Based on Osmotic Pressure Principle
- PMID: 35629669
- PMCID: PMC9143235
- DOI: 10.3390/ma15103643
Study on Preparation of Polymer-Modified Bentonite and Sand Mixtures Based on Osmotic Pressure Principle
Abstract
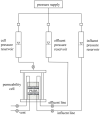
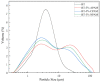
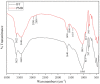
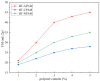
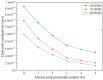
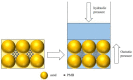
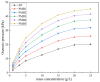
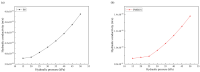
Polymer-modified bentonite and sand mixtures (PMBS) are widely used in the engineering field due to their low cost and low permeability. In this study, different ionic types of polyacrylamides were used to modify bentonite to improve its swelling properties and impermeability. The physicochemical properties of polymer-modified bentonite were characterized by X-ray diffraction, particle size distribution, IR spectroscopy, SEM, and free swell index (FSI) to further demonstrate the successful organic modification of bentonite. To investigate the impermeability mechanism of PMBS from the perspective of osmotic pressure, the colloidal osmotic pressure of bentonite and hydraulic conductivity were compared. The results showed that anionic polyacrylamide (APAM) had the most obvious improvement on the swelling properties of bentonite, and 3% APAM increased the FSI of bentonite from 15 mL/2 g to 41 mL/2 g. With the increase in polymer dosage, the colloidal osmotic pressure of bentonite increased and the hydraulic conductivity of PMBS decreased significantly. The interior of PMBS is equivalent to a highly concentrated bentonite-sand-water system. When the colloidal osmotic pressure in the restricted space is higher than the external hydraulic pressure, it will prevent infiltration from occurring. When the external hydraulic pressure exceeds the high concentration of bentonite colloid osmotic pressure, the hydraulic conductivity may increase rapidly. Therefore, the impermeability of PMBS depends on the colloidal osmotic pressure of bentonite. Finally, it was confirmed that PMBS had a self-healing capacity by simulating damage to PMBS.
Keywords: bentonite; hydraulic conductivity; osmotic pressure; polymer-modified; self-healing; swell index.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures





Similar articles
-
A Study on the Impermeability of Nanodispersible Modified Bentonite Based on Colloidal Osmotic Pressure Mechanisms and the Adsorption of Harmful Substances.Nanomaterials (Basel). 2023 Jun 11;13(12):1840. doi: 10.3390/nano13121840. Nanomaterials (Basel). 2023. PMID: 37368270 Free PMC article.
-
Modification of bentonite with black cotton soil and carboxyl methyl cellulose for the enhancement of hydraulic performance of geosynthetic clay liners.Water Sci Technol. 2024 Apr;89(7):1846-1859. doi: 10.2166/wst.2024.093. Epub 2024 Mar 25. Water Sci Technol. 2024. PMID: 38619907
-
Hydraulic performance, consolidation characteristics and shear strength analysis of bentonites in the presence of fly-ash, sewage sludge and paper-mill leachates for landfill application.J Environ Manage. 2022 Jan 15;302(Pt A):113977. doi: 10.1016/j.jenvman.2021.113977. Epub 2021 Oct 20. J Environ Manage. 2022. PMID: 34688046
-
Saturated hydraulic conductivity of compacted steel slag-bentonite mixtures--A potential hydraulic barrier material of landfill cover.Waste Manag. 2022 May 1;144:349-356. doi: 10.1016/j.wasman.2022.04.004. Epub 2022 Apr 15. Waste Manag. 2022. PMID: 35436714
-
Long term chemo-hydro-mechanical behavior of compacted soil bentonite polymer complex submitted to synthetic leachate.Waste Manag. 2016 Jul;53:92-104. doi: 10.1016/j.wasman.2016.04.023. Epub 2016 May 2. Waste Manag. 2016. PMID: 27156365 Review.
Cited by
-
A Study on the Impermeability of Nanodispersible Modified Bentonite Based on Colloidal Osmotic Pressure Mechanisms and the Adsorption of Harmful Substances.Nanomaterials (Basel). 2023 Jun 11;13(12):1840. doi: 10.3390/nano13121840. Nanomaterials (Basel). 2023. PMID: 37368270 Free PMC article.
References
-
- Ding Y., Zhao J., Liu J., Zhou J., Cheng L., Zhao J., Shao Z., Iris Ç., Pan B., Li X., et al. A Review of China’s Municipal Solid Waste (MSW) and Comparison with International Regions: Management and Technologies in Treatment and Resource Utilization. J. Clean. Prod. 2021;293:126144. doi: 10.1016/j.jclepro.2021.126144. - DOI
-
- Fernández-González J.M., Grindlay A.L., Serrano-Bernardo F., Rodríguez-Rojas M.I., Zamorano M. Economic and Environmental Review of Waste-to-Energy Systems for Municipal Solid Waste Management in Medium and Small Municipalities. Waste Manag. 2017;67:360–374. doi: 10.1016/j.wasman.2017.05.003. - DOI - PubMed
-
- Zhao Q., Choo H., Bhatt A., Burns S.E., Bate B. Review of the Fundamental Geochemical and Physical Behaviors of Organoclays in Barrier Applications. Appl. Clay Sci. 2017;142:2–20. doi: 10.1016/j.clay.2016.11.024. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

