Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA-A Receptors
- PMID: 35629767
- PMCID: PMC9146934
- DOI: 10.3390/membranes12050440
Xenopus Oocytes: A Tool to Decipher Molecular Specificity of Insecticides towards Mammalian and Insect GABA-A Receptors
Abstract
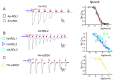
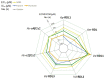
The number of insect GABA receptors (GABAr) available for expression studies has been recently increased by the cloning of the Acyrthosiphon pisum (pea aphid) RDL subunits. This large number of cloned RDL subunits from pest and beneficial insects opens the door to parallel pharmacological studies on the sensitivity of these different insect GABAr to various agonists or antagonists. The resulting analysis of the molecular basis of the species-specific GABAr responses to insecticides is necessary not only to depict and understand species toxicity, but also to help at the early identification of unacceptable toxicity of insecticides toward beneficial insects such as Apis mellifera (honeybees). Using heterologous expression in Xenopus laevis oocytes, and two-electrode voltage-clamp recording to assess the properties of the GABAr, we performed a comparative analysis of the pharmacological sensitivity of RDL subunits from A. pisum, A. mellifera and Varroa destructor GABAr to three pesticides (fipronil, picrotoxin and dieldrin). These data were compared to similar characterizations performed on two Homo sapiens GABA-A receptors (α2β2γ2 and α2β2γ2). Our results underline a global conservation of the pharmacological profiles of these receptors, with some interesting species specificities, nonetheless, and suggest that this approach can be useful for the early identification of poorly specific molecules.
Keywords: heterologous expression; human; pharmacology; phytopharmaceuticals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ozoe Y. g-Aminobutyrate- and Glutamate-gated Chloride Channels as Targets of Insecticides. In: Simpson S.J., Casas J., editors. Advances in Insect Physiology. Volume 44. Elsevier Ltd.; Amsterdam, The Netherlands: 2013. pp. 211–286. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

