Ultrafiltration Membranes Functionalized with Copper Oxide and Zwitterions for Fouling Resistance
- PMID: 35629870
- PMCID: PMC9145826
- DOI: 10.3390/membranes12050544
Ultrafiltration Membranes Functionalized with Copper Oxide and Zwitterions for Fouling Resistance
Abstract
Polymeric membrane fouling is a long-standing challenge for water filtration. Metal/metal oxide nanoparticle functionalization of the membrane surface can impart anti-fouling properties through the reactivity of the metal species and the generation of radical species. Copper oxide nanoparticles (CuO NPs) are effective at reducing organic fouling when used in conjunction with hydrogen peroxide, but leaching of copper ions from the membrane has been observed, which can hinder the longevity of the CuO NP activity at the membrane surface. Zwitterions can reduce organic fouling and stabilize NP attachment, suggesting a potential opportunity to combine the two functionalizations. Here, we coated polyethersulfone (PES) ultrafiltration membranes with polydopamine (PDA) and attached the zwitterionic compound, thiolated 2-methacryloyloxyethyl phosphorylcholine (MPC-SH), and CuO NPs. Functionalized membranes resulted in a higher flux recovery ratio (0.694) than the unfunctionalized PES control (0.599). Copper retention was high (>96%) for functionalized membranes. The results indicate that CuO NPs and MPC-SH can reduce organic fouling with only limited copper leaching.
Keywords: anti-fouling; copper oxide; polydopamine; ultrafiltration; zwitterion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







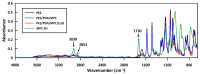
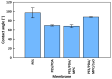

References
-
- Gao W., Liang H., Ma J., Han M., Chen Z., Han Z., Li G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination. 2011;272:1–8. doi: 10.1016/j.desal.2011.01.051. - DOI
-
- Mansouri J., Harrisson S., Chen V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010;20:4567–4586. doi: 10.1039/b926440j. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

