The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets
- PMID: 35629964
- PMCID: PMC9147253
- DOI: 10.3390/metabo12050460
The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets
Abstract
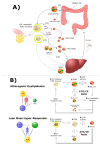
When lean people adopt carbohydrate-restricted diets (CRDs), they may develop a lipid profile consisting of elevated LDL-cholesterol (LDL-C) and HDL-cholesterol (HDL-C) with low triglycerides (TGs). The magnitude of this lipid profile correlates with BMI such that those with lower BMI exhibit larger increases in both LDL-C and HDL-C. The inverse association between BMI and LDL-C and HDL-C change on CRD contributed to the discovery of a subset of individuals-termed Lean Mass Hyper-Responders (LMHR)-who, despite normal pre-diet LDL-C, as compared to non-LMHR (mean levels of 148 and 145 mg/dL, respectively), exhibited a pronounced hyperlipidemic response to a CRD, with mean LDL-C and HDL-C levels increasing to 320 and 99 mg/dL, respectively, in the context of mean TG of 47 mg/dL. In some LMHR, LDL-C levels may be in excess of 500 mg/dL, again, with relatively normal pre-diet LDL-C and absent of genetic findings indicative of familial hypercholesterolemia in those who have been tested. The Lipid Energy Model (LEM) attempts to explain this metabolic phenomenon by positing that, with carbohydrate restriction in lean persons, the increased dependence on fat as a metabolic substrate drives increased hepatic secretion and peripheral uptake of TG contained within very low-density lipoproteins (VLDL) by lipoprotein lipase, resulting in marked elevations of LDL-C and HDL-C, and low TG. Herein, we review the core features of the LEM. We review several existing lines of evidence supporting the model and suggest ways to test the model's predictions.
Keywords: HDL-cholesterol; LDL-cholesterol; VLDL-cholesterol; carbohydrate restriction; lean mass hyper-responder; lipoprotein lipase; triglyceride-rich lipoproteins.
Conflict of interest statement
N.G.N. is coauthor of a Mediterranean low-carbohydrate diet cookbook; he donates all royalty payments to nutrition research and education. D.S.L. received royalties for books that recommend a carbohydrate-modified diet, and his spouse owns a nutrition education and consulting business. A.K. is an author of a patent EP17306042 entitled “Novel Assay of HDL Function.” D.F. receives financial contributions from membership (e.g., through Patreon) for continued research; and is a partner in Own Your Labs LLC, with all proceeds contributed to the Citizen Science Foundation. A.S.-M. reports no relationship.
Figures


References
-
- Valsdottir T.D., Henriksen C., Odden N., Nellemann B., Jeppesen P.B., Hisdal J., Westerberg A.C., Jensen J. Effect of a Low-Carbohydrate High-Fat Diet and a Single Bout of Exercise on Glucose Tolerance, Lipid Profile and Endothelial Function in Normal Weight Young Healthy Females. Front. Physiol. 2019;10:1499. doi: 10.3389/fphys.2019.01499. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

