From Soft to Hard Biomimetic Materials: Tuning Micro/Nano-Architecture of Scaffolds for Tissue Regeneration
- PMID: 35630247
- PMCID: PMC9144100
- DOI: 10.3390/mi13050780
From Soft to Hard Biomimetic Materials: Tuning Micro/Nano-Architecture of Scaffolds for Tissue Regeneration
Abstract
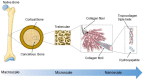
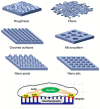
Failure of tissues and organs resulting from degenerative diseases or trauma has caused huge economic and health concerns around the world. Tissue engineering represents the only possibility to revert this scenario owing to its potential to regenerate or replace damaged tissues and organs. In a regeneration strategy, biomaterials play a key role promoting new tissue formation by providing adequate space for cell accommodation and appropriate biochemical and biophysical cues to support cell proliferation and differentiation. Among other physical cues, the architectural features of the biomaterial as a kind of instructive stimuli can influence cellular behaviors and guide cells towards a specific tissue organization. Thus, the optimization of biomaterial micro/nano architecture, through different manufacturing techniques, is a crucial strategy for a successful regenerative therapy. Over the last decades, many micro/nanostructured biomaterials have been developed to mimic the defined structure of ECM of various soft and hard tissues. This review intends to provide an overview of the relevant studies on micro/nanostructured scaffolds created for soft and hard tissue regeneration and highlights their biological effects, with a particular focus on striated muscle, cartilage, and bone tissue engineering applications.
Keywords: architectural features; bone regeneration; cardiac muscular regeneration; cartilage regeneration; micro/nanostructured biomaterials; scaffolding strategies; tissue engineering; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5:686–705. doi: 10.1038/s41578-020-0209-x. - DOI
-
- Sato Y., Bando H., Di Piazza M., Gowing G., Herberts C., Jackman S., Leoni G., Libertini S., MacLachlan T., McBlane J.W., et al. Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy. 2019;21:1095–1111. doi: 10.1016/j.jcyt.2019.10.001. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

