Oxonitrogenated Derivatives of Eremophilans and Eudesmans: Antiproliferative and Anti- Trypanosoma cruzi Activity
- PMID: 35630539
- PMCID: PMC9143450
- DOI: 10.3390/molecules27103067
Oxonitrogenated Derivatives of Eremophilans and Eudesmans: Antiproliferative and Anti- Trypanosoma cruzi Activity
Abstract
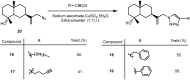
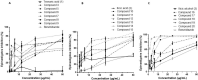
Cancer is one of the most important causes of death worldwide. Solid tumors represent the vast majority of cancers (>90%), and the chemotherapeutic agents used for their treatment are still characterized by variable efficacy and toxicity. Sesquiterpenes are a group of natural compounds that have shown a wide range of biological activities, including cytotoxic and antiparasitic activity, among others. The antiproliferative activity of natural sesquiterpenes, tessaric acid, ilicic acid, and ilicic alcohol and their semisynthetic derivatives against HeLa, T-47D, WiDr, A549, HBL-100, and SW1573 cell lines were evaluated. The effect of the compounds on Trypanosoma cruzi epimastigotes was also assessed. The selectivity index was calculated using murine splenocytes. Derivatives 13 and 15 were the most antiproliferative compounds, with GI50 values ranging between 5.3 (±0.32) and 14 (±0.90) μM, in all cell lines tested. The presence of 1,2,3-triazole groups in derivatives 15−19 led to improvements in activity compared to those corresponding to the starting natural product (3), with GI50 values ranging between 12 (±1.5) and 17 (±1.1) μM and 16 being the most active compound. In relation to the anti-T. cruzi activity, derivatives 7 and 16 obtained from tessaric acid and ilicic acid were among the most active and selective compounds with IC50 values of 9.3 and 8.8 µM (SI = 8.0 and 9.4), respectively.
Keywords: Asteraceae; Trypanosoma cruzi; antiproliferative activity; ilicic acid; ilicic alcohol; sesquiterpenes; tessaric acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

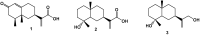





References
-
- Organización Mundial de la Salud (OMS) Paludismo Tratamiento del Paludismo: Panorama General. 2018. [(accessed on 24 May 2019)]. Available online: https://www.who.int/malaria/areas/treatment/overview/es/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

