Synthesis of Silica-Based Quaternized Adsorption Material and Study on Its Adsorption Behavior for Pu(IV)
- PMID: 35630589
- PMCID: PMC9145714
- DOI: 10.3390/molecules27103110
Synthesis of Silica-Based Quaternized Adsorption Material and Study on Its Adsorption Behavior for Pu(IV)
Abstract
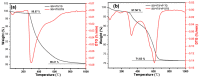
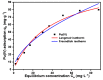
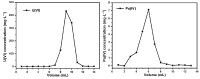
In this research, we explored the synthesis optimization of the silica-based quaternized adsorption material (SG-VTS-VPQ) and its adsorption behavior for Pu(IV). By optimizing the synthesis process, the grafting amount of 4-vinylpyridine reached 1.25 mmol·g-1. According to the analysis of NMR and XPS, the quaternization rate of pyridine groups reached 91.13%. In the adsorption experiments, the thermodynamic experiment results show that the adsorption of Pu(IV) by SG-VTS-VPQ is more in line with the Langmuir adsorption model and the adsorption type is a typical chemical adsorption; the kinetic results show that adsorption process is more in line with the pseudo first-order kinetic model, and the larger specific surface area of SG-VTS-VPQ plays an important role in the adsorption. The results of the adsorption mechanism show that the adsorption of Pu(IV) by SG-VTS-VPQ is mainly complex anion Pu(NO3)62- and Pu(NO3)5-. This research provides in-depth and detailed ideas for the surface modification and application of porous silica gel, and at the same time provides a new way to develop the direction of the analysis of pretreatment materials in the spent fuel reprocessing field.
Keywords: Pu(IV); adsorption; grafting; reaction mechanism; reprocessing; silica gel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















References
-
- Govindan P., Vijayan K., Kumar G. Recovery and purification of plutonium in lean plutonium product stream obtained during reprocessing of fast reactor spent fuels. J. Radioanal. Nucl. Chem. 2013;295:307–313. doi: 10.1007/s10967-012-2194-z. - DOI
-
- Pathak S., Pius I., Mukerjee S. Studies on sorption of plutonium from carbonate medium on polyacrylhydroxamic acid resin. J. Radioanal. Nucl. Chem. 2013;293:483–488. doi: 10.1007/s10967-012-1787-x. - DOI
-
- Eitrheim E., Knight A., Andrew B. Separation of gallium and actinides in plutonium nuclear materials by extraction chromatography. J. Radioanal. Nucl. Chem. 2014;303:123–130. doi: 10.1007/s10967-014-3310-z. - DOI
-
- Ryan J. Concentration and final purification of neptunium by anion exchange. Off. Sci. Tech. Inf. 1959;6:13–29.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

