The Relationship between the IC50 Values and the Apparent Inhibition Constant in the Study of Inhibitors of Tyrosinase Diphenolase Activity Helps Confirm the Mechanism of Inhibition
- PMID: 35630619
- PMCID: PMC9142954
- DOI: 10.3390/molecules27103141
The Relationship between the IC50 Values and the Apparent Inhibition Constant in the Study of Inhibitors of Tyrosinase Diphenolase Activity Helps Confirm the Mechanism of Inhibition
Abstract
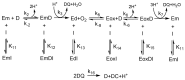
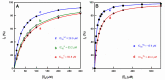
Tyrosinase is the enzyme involved in melanization and is also responsible for the browning of fruits and vegetables. Control of its activity can be carried out using inhibitors, which is interesting in terms of quantitatively understanding the action of these regulators. In the study of the inhibition of the diphenolase activity of tyrosinase, it is intriguing to know the strength and type of inhibition. The strength is indicated by the value of the inhibition constant(s), and the type can be, in a first approximation: competitive, non-competitive, uncompetitive and mixed. In this work, it is proposed to calculate the degree of inhibition (iD), varying the concentration of inhibitor to a fixed concentration of substrate, L-dopa (D). The non-linear regression adjustment of iD with respect to the initial inhibitor concentration [I]0 allows for the calculation of the inhibitor concentration necessary to inhibit the activity by 50%, at a given substrate concentration (IC50), thus avoiding making interpolations between different values of iD. The analytical expression of the IC50, for the different types of inhibition, are related to the apparent inhibition constant (KIapp). Therefore, this parameter can be used: (a) To classify a series of inhibitors of an enzyme by their power. Determining these values at a fixed substrate concentration, the lower IC50, the more potent the inhibitor. (b) Checking an inhibitor for which the type and the inhibition constant have been determined (using the usual methods), must confirm the IC50 value according to the corresponding analytical expression. (c) The type and strength of an inhibitor can be analysed from the study of the variation in iD and IC50 with substrate concentration. The dependence of IC50 on the substrate concentration allows us to distinguish between non-competitive inhibition (iD does not depend on [D]0) and the rest. In the case of competitive inhibition, this dependence of iD on [D]0 leads to an ambiguity between competitive inhibition and type 1 mixed inhibition. This is solved by adjusting the data to the possible equations; in the case of a competitive inhibitor, the calculation of KI1app is carried out from the IC50 expression. The same occurs with uncompetitive inhibition and type 2 mixed inhibition. The representation of iD vs. n, with n=[D]0/KmD, allows us to distinguish between them. A hyperbolic iD vs. n representation that passes through the origin of coordinates is a characteristic of uncompetitive inhibition; the calculation of KI2app is immediate from the IC50 value. In the case of mixed inhibitors, the values of the apparent inhibition constant of meta-tyrosinase (Em) and oxy-tyrosinase (Eox), KI1app and the apparent inhibition constant of metatyrosinase/Dopa complexes (EmD) and oxytyrosinase/Dopa (EoxD), KI2app are obtained from the dependence of iD vs. n, and the results obtained must comply with the IC50 value.
Keywords: IC50; K I app; diphenolase activity; inhibition; polyphenol oxidase; tyrosinase.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



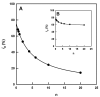
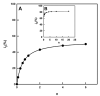
References
-
- Maddaleno A.S., Camargo J., Mitjans M., Vinardell M.P. Melanogenesis and Melasma Treatment. Cosmetics. 2021;8:82. doi: 10.3390/cosmetics8030082. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

