Impact of the Hydrolysis and Methanolysis of Bidesmosidic Chenopodium quinoa Saponins on Their Hemolytic Activity
- PMID: 35630692
- PMCID: PMC9144749
- DOI: 10.3390/molecules27103211
Impact of the Hydrolysis and Methanolysis of Bidesmosidic Chenopodium quinoa Saponins on Their Hemolytic Activity
Abstract
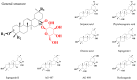
Saponins are specific metabolites abundantly present in plants and several marine animals. Their high cytotoxicity is associated with their membranolytic properties, i.e., their propensity to disrupt cell membranes upon incorporation. As such, saponins are highly attractive for numerous applications, provided the relation between their molecular structures and their biological activities is understood at the molecular level. In the present investigation, we focused on the bidesmosidic saponins extracted from the quinoa husk, whose saccharidic chains are appended on the aglycone via two different linkages, a glycosidic bond, and an ester function. The later position is sensitive to chemical modifications, such as hydrolysis and methanolysis. We prepared and characterized three sets of saponins using mass spectrometry: (i) bidesmosidic saponins directly extracted from the ground husk, (ii) monodesmosidic saponins with a carboxylic acid group, and (iii) monodesmosidic saponins with a methyl ester function. The impact of the structural modifications on the membranolytic activity of the saponins was assayed based on the determination of their hemolytic activity. The natural bidesmosidic saponins do not present any hemolytic activity even at the highest tested concentration (500 µg·mL-1). Hydrolyzed saponins already degrade erythrocytes at 20 µg·mL-1, whereas 100 µg·mL-1 of transesterified saponins is needed to induce detectable activity. The observation that monodesmosidic saponins, hydrolyzed or transesterified, are much more active against erythrocytes than the bidesmosidic ones confirms that bidesmosidic saponins are likely to be the dormant form of saponins in plants. Additionally, the observation that negatively charged saponins, i.e., the hydrolyzed ones, are more hemolytic than the neutral ones could be related to the red blood cell membrane structure.
Keywords: chemical modification; hemolytic activity; mass spectrometry; saponins; structure-activity relationship.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Rohit Dutt A.K., Sharma R.K., Keservani V.G. Promising Drug Molecules of Natural Origin. CRC Press; Coca Raton, FL, USA: 2020.
-
- Hostettmann K., Marston A. Chemistry Pharmacology of Natural Products-Saponins. Cambridge University Press; Cambridge, UK: 2005.
-
- Deacon B.J.W., Mitchell R.T. Toxicity of oat roots, oat root extracts, and saponins to zoospores of Pythium spp. and other fungi. Trans. Br. Mycol. Soc. 1985;84:479–487. doi: 10.1016/S0007-1536(85)80010-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

