Preparation and Characterization of Controlled-Release Floating Bilayer Tablets of Esomeprazole and Clarithromycin
- PMID: 35630719
- PMCID: PMC9143198
- DOI: 10.3390/molecules27103242
Preparation and Characterization of Controlled-Release Floating Bilayer Tablets of Esomeprazole and Clarithromycin
Abstract
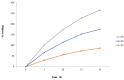
Controlled-release effervescent floating bilayer tablets reduce dosage frequency and improve patient compliance with enhanced therapeutic outcomes. Generally, two different tablets of clarithromycin and esomeprazole, respectively, are given for the treatment of Helicobacter pylori infection and it might be worth incorporating both in a single tablet. In the current study, controlled-release floating bilayer tablets of clarithromycin and esomeprazole (F1−F4) were developed with different rates of polymeric materials by a direct compression method. During the formulation, Fourier-transform infrared spectroscopy (FTIR) analysis was performed for possible interactions between drugs and excipients. No interactions between drugs and excipients were noted. Moreover, the bilayer tablets’ thickness, diameter, friability, hardness, weight variation, dissolution, and percent purity were found within the acceptable limits. The floating lag time and total floating time of all formulations were found to be < 25 s and 24 h, respectively. The release of both the clarithromycin and esomeprazole started at the same time from the controlled-release floating bilayer tablets by anomalous non-Fickian diffusion, and the polymeric materials extended the drug release rate up to 24 h. In the case of F1, the results approached ideal zero-order kinetics. The dissolution profiles of the tested and reference tablet formulations were compared, but no significant differences were observed. It can be concluded that such controlled-release effervescent floating bilayer tablets can be efficiently used in clinical practice to reduce dosage frequency and increase patient compliance with continuous drug release for 24 h, which ultimately might enhance therapeutic efficacy.
Keywords: clarithromycin; esomeprazole; floating bilayer tablets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sharma V., Rathore D.S., Kumar A. Floating Drug Delivery System: A Review. Int. J. Med. Biomed. Stud. 2020;4:23–30. doi: 10.32553/ijmbs.v4i8.1333. - DOI
-
- Devendiran B., Mothilal M., Damodharan N. Floating Drug Delivery an Emerging Technology with Promising Market value. Res. J. Pharm. Technol. 2020;13:3014–3020. doi: 10.5958/0974-360X.2020.00533.8. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

